Assembly and processing of human immunodeficiency virus Gag mutants containing a partial replacement of the matrix domain by the viral protease domain
- PMID: 10708461
- PMCID: PMC111845
- DOI: 10.1128/jvi.74.7.3418-3422.2000
Assembly and processing of human immunodeficiency virus Gag mutants containing a partial replacement of the matrix domain by the viral protease domain
Abstract
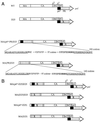
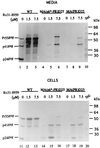
We constructed human immunodeficiency virus (HIV) mutants by replacing the matrix domain with sequences encoding the viral protease or p6* and protease. The chimeras retaining matrix myristylation and processing signals underwent efficient autoprocessing with severely defective particle budding. The budding defects of the chimeras were rescued by suppressing the chimera protease activity either through addition of an HIV protease inhibitor or through inactivating the chimera protease via a substitution mutation of the catalytic aspartic acid residue. This resulted in the release of chimeric virus-like particles with the density of a wild-type retrovirus particle. In addition, the assembly-competent but processing-defective chimeras produced proteolytically processed particles with significant reverse transcriptase activity when a downstream native pol gene was present. These results suggest that HIV has the potential to adapt heterologous sequences in place of the matrix sequence without major effects on virus-like particle budding. In addition, the positions of the protease and substrate accessibility may contribute significantly toward avoiding a premature Gag or Gag-Pol process, which leads to severe defects in both particle budding and incorporation.
Figures


References
-
- Chen Y-L, Ts'ai P-W, Yang C-C, Wang C-T. Generation of infectious virus particles by transient co-expression of human immunodeficiency virus type 1 gag mutants. J Gen Virol. 1997;78:2497–2501. - PubMed
-
- Freed E O. HIV Gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. - PubMed
-
- Gelderblom H R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991;5:617–638. - PubMed
-
- Henderson L E, Bowers M A, Sowder II R C, Serabyn S A, Johnson D G, Bess J W, Jr, Arthur L O, Bryant D K, Fenselau C. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processing, and complete amino acid sequences. J Virol. 1992;66:1856–1865. - PMC - PubMed
-
- Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

