A new approach for the identification and cloning of genes: the pBACwich system using Cre/lox site-specific recombination
- PMID: 10710436
- PMCID: PMC102802
- DOI: 10.1093/nar/28.7.e19
A new approach for the identification and cloning of genes: the pBACwich system using Cre/lox site-specific recombination
Abstract
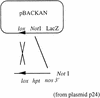
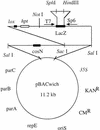
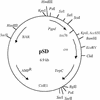
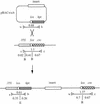
With current plant transformation methods ( Agrobacterium, biolistics and protoplast fusion), insertion of DNA into the genome occurs randomly and in many instances at multiple sites. Associated position effects, copy number differences and multigene interactions can make gene expression experiments difficult to interpret and plant phenotypes less predictable. An alternative approach to random integration of large DNA fragments into plants is to utilize one of several site-specific recombination (SSR) systems, such as Cre/ lox. Cre has been shown in numerous instances to mediate lox site-specific recombination in animal and plant cells. By incorporating the Cre/ lox SSR system into a bacterial artificial chromosome (BAC) vector, a more precise evaluation of large DNA inserts for genetic complementation should be possible. Site-specific insertion of DNA into predefined sites in the genome may eliminate unwanted 'position effects' caused by the random integration of exogenously introduced DNA. In an effort to make the Cre/ lox system an effective tool for site-directed integration of large DNAs, we constructed and tested a new vector potentially capable of integrating large DNA inserts into plant and fungal genomes. In this study, we present the construction of a new BAC vector, pBACwich, for the system and the use of this vector to demonstrate SSR of large DNA inserts (up to 230 kb) into plant and fungal genomes.
Figures



References
-
- van Haaren M.J. and Ow,D.W. (1993) Plant Mol. Biol., 23, 525–533. - PubMed
-
- Riordan J.R., Rommens,J.M., Kerem,B., Alon,N., Rozmahel,R., Grzelczak,Z., Zielenski,J., Lok,S., Plavsic,N. and Chou,J.L. (1989) Science, 245, 1066–1073. - PubMed
-
- Rommens J.M., Iannuzzi,M.C., Kerem,B., Drumm,M.L., Melmer,G., Dean,M., Rozmahel,R., Cole,J.L., Kennedy,D., Hidaka,N. et al. (1989) Science, 245, 1059–1065. - PubMed
-
- Collins F.S. (1990) Harvey Lect., 86, 149–164. - PubMed
-
- van Bokhoven H., van den Hurk,J.A., Bogerd,L., Philippe,C., Gilgenkrantz,S., de Jong,P., Ropers,H.H. and Cremers,F.P. (1994) Hum. Mol. Genet., 3, 1041–1046. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

