Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein
- PMID: 10712202
- PMCID: PMC1288169
- DOI: 10.1086/302829
Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein
Abstract
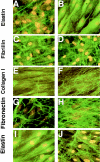
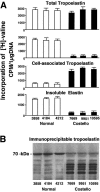
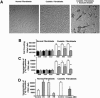
Costello syndrome is characterized by mental retardation, loose skin, coarse face, skeletal deformations, cardiomyopathy, and predisposition to numerous malignancies. The genetic origin of Costello syndrome has not yet been defined. Using immunohistochemistry and metabolic labeling with [3H]-valine, we have established that cultured skin fibroblasts obtained from patients with Costello syndrome did not assemble elastic fibers, despite an adequate synthesis of tropoelastin and normal deposition of the microfibrillar scaffold. We found that impaired production of elastic fibers by these fibroblasts is associated with a functional deficiency of the 67-kD elastin-binding protein (EBP), which is normally required to chaperone tropoelastin through the secretory pathways and to its extracellular assembly. Metabolic pulse labeling of the 67-kD EBP with radioactive serine and further chase of this tracer indicated that both normal fibroblasts and fibroblasts from patients with Costello syndrome initially synthesized comparable amounts of this protein; however, the fibroblasts from Costello syndrome patients quickly lost it into the conditioned media. Because the normal association between EBP and tropoelastin can be disrupted on contact with galactosugar-bearing moieties, and the fibroblasts from patients with Costello syndrome revealed an unusual accumulation of chondroitin sulfate-bearing proteoglycans (CD44 and biglycan), we postulate that a chondroitin sulfate may be responsible for shedding EBP from Costello cells and in turn for their impaired elastogenesis. This was further supported by the fact that exposure to chondroitinase ABC, an enzyme capable of chondroitin sulfate degradation, restored normal production of elastic fibers by fibroblasts from patients with Costello syndrome. We also present evidence that loss of EBP from fibroblasts of Costello syndrome patients is associated with an unusually high rate of cellular proliferation.
Figures

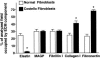


References
Electronic-Database Information
-
- Online Mendelian Inheritance in Man (OMIM): http://www.ncbi.nlm.nih.gov/Omim (for Costello syndrome [MIM 218040])
References
-
- Belcher RW (1972) Ultrastructure of the skin in the genetic mucopolysaccharidoses. Arch Pathol 94:511–518 - PubMed
-
- Borochowitz Z, Pavone L, Mazor G, Rizzo R, Dar H (1992) New mental congenital anomalies: mental retardation syndrome (MR/MCA) with facio-cutaneous-skeletal involvement. Am J Med Genet 43:678–685 - PubMed
-
- Chiu RK, Droll A, Dougherty ST, Carpenito C, Cooper DL, Dougherty GJ (1999) Alternatively spliced CD44 isoforms containing exon v10 promote cellular adhesion through the recognition of chondroitin sulfate–modified CD44. Exp Cell Res 248:314–321 - PubMed
-
- Christiano AM, Uitto J (1994) Molecular pathology of elastic fibers. J Invest Dermatol Suppl 103:53S–57S - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

