The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients
- PMID: 10712431
- PMCID: PMC289174
- DOI: 10.1172/JCI8047
The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients
Abstract
Mutations in Notch3 cause CADASIL (cerebral autosomal dominant adult onset arteriopathy), which leads to stroke and dementia in humans. CADASIL arteriopathy is characterized by major alterations of vascular smooth muscle cells and the presence of specific granular osmiophilic deposits. Patients carry highly stereotyped mutations that lead to an odd number of cysteine residues within EGF-like repeats of the Notch3 receptor extracellular domain. Such mutations may alter the processing or the trafficking of this receptor, or may favor its oligomerization. In this study, we examined the Notch3 expression pattern in normal tissues and investigated the consequences of mutations on Notch3 expression in transfected cells and CADASIL brains. In normal tissues, Notch3 expression is restricted to vascular smooth muscle cells. Notch3 undergoes a proteolytic cleavage leading to a 210-kDa extracellular fragment and a 97-kDa intracellular fragment. In CADASIL brains, we found evidence of a dramatic and selective accumulation of the 210-kDa Notch3 cleavage product. Notch3 accumulates at the cytoplasmic membrane of vascular smooth muscle cells, in close vicinity to but not within the granular osmiophilic material. These results strongly suggest that CADASIL mutations specifically impair the clearance of the Notch3 ectodomain, but not the cytosolic domain, from the cell surface.
Figures



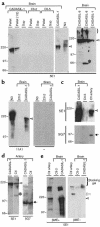

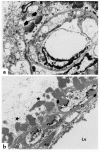
Comment in
-
CADASIL: Notch signaling defect or protein accumulation problem?J Clin Invest. 2000 Mar;105(5):561-2. doi: 10.1172/JCI9511. J Clin Invest. 2000. PMID: 10712425 Free PMC article. No abstract available.
References
-
- Joutel A, et al. Notch3 mutations in CADASIL, an hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. - PubMed
-
- Tournier-Lasserve E, Iba-Zizen MT, Romero N, Bousser MG. Autosomal dominant syndrome with stroke-like episodes and leukoencephalopathy. Stroke. 1991;22:1297–1302. - PubMed
-
- Tournier-Lasserve E, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy maps to chromosome 19q12. Nat Genet. 1993;3:256–259. - PubMed
-
- Chabriat H, et al. Clinical spectrum of CADASIL: a study of 7 families. Lancet. 1995;346:934–939. - PubMed
-
- Baudrimont M, Dubas F, Joutel A, Tournier-Lasserve E, Bousser MG. Autosomal dominant leukoencephalopathy and subcortical ischemic stroke. A clinicopathological study. Stroke. 1993;24:122–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

