Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus
- PMID: 10712434
- PMCID: PMC292455
- DOI: 10.1172/JCI8748
Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus
Abstract
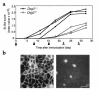
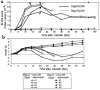
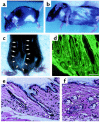
The development of experimental models of active autoimmune diseases can be difficult due to tolerance of autoantigens, but knockout mice, which fail to acquire tolerance to the defective gene product, provide a useful tool for this purpose. Using knockout mice lacking desmoglein 3 (Dsg3), the target antigen of pemphigus vulgaris (PV), we have generated an active disease model for this autoantibody-mediated disease. Dsg3(-/-) mice, but not Dsg3(+/-) littermates, produced anti-Dsg3 IgG that binds native Dsg3, when immunized with recombinant mouse Dsg3. Splenocytes from the immunized Dsg3(-/-) mice were then adoptively transferred into Rag-2(-/-) immunodeficient mice expressing Dsg3. Anti-Dsg3 IgG was stably produced in the recipient mice for more than 6 months without further boosting. This IgG bound to Dsg3 in vivo and disrupted the cell-cell adhesion of keratinocytes. Consequently, the recipient mice developed erosions in their oral mucous membranes with typical histologic findings of PV. In addition, the recipient mice showed telogen hair loss, as found in Dsg3(-/-) mice. Collectively, the recipient mice developed the phenotype of PV due to the pathogenic anti-Dsg3 IgG. This model will be valuable for developing novel therapeutic strategies. Furthermore, our approach can be applied broadly for the development of various autoimmune disease models.
Figures

References
-
- MacDonald HR. Mechanisms of immunological tolerance. Science. 1989;246:982. - PubMed
-
- Bach JF, Koutouzov S, van Endert PM. Are there unique autoantigens triggering autoimmune diseases? Immunol Rev. 1998;164:139–155. - PubMed
-
- Stanley, J.R. 1998. Pemphigus. In Dermatology in general medicine. I.M. Freedberg et al., editors. McGraw-Hill. New York, NY. 654–666.
-
- Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

