Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state
- PMID: 10712502
- PMCID: PMC14813
- DOI: 10.1091/mbc.11.3.819
Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state
Abstract
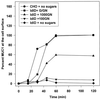
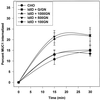
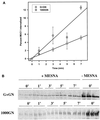
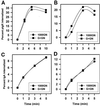
MUC1 is a mucin-like type 1 transmembrane protein associated with the apical surface of epithelial cells. In human tumors of epithelial origin MUC1 is overexpressed in an underglycosylated form with truncated O-glycans and accumulates in intracellular compartments. To understand the basis for this altered subcellular localization, we compared the synthesis and trafficking of various glycosylated forms of MUC1 in normal (Chinese hamster ovary) cells and glycosylation-defective (ldlD) cells that lack the epimerase to make UDP-Gal/GalNAc from UDP-Glc/GlcNAc. Although the MUC1 synthesized in ldlD cells was rapidly degraded, addition of GalNAc alone to the culture media resulted in stabilization and near normal surface expression of MUC1 with truncated but sialylated O-glycans. Interestingly, the initial rate of endocytosis of this underglycosylated MUC1 was stimulated by twofold compared with fully glycosylated MUC1. However, the half-lives of the two forms were not different, indicating that trafficking to lysosomes was not affected. Both the normal and stimulated internalization of MUC1 could be blocked by hypertonic media, a hallmark of clathrin-mediated endocytosis. MUC1 endocytosis was also blocked by expression of a dominant-negative mutant of dynamin-1 (K44A), and MUC1 was observed in both clathrin-coated pits and vesicles by immunoelectron microscopy of ultrathin cryosections. Our data suggest that the subcellular redistribution of MUC1 in tumor cells could be a direct result of altered endocytic trafficking induced by its aberrant glycosylation; potential models are discussed. These results also implicate a new role for O-glycans on mucin-like membrane proteins entering the endocytic pathway through clathrin-coated pits.
Figures





References
-
- Altschuler Y, Poland PA, Kinlough CL, Hughey RP. Role of endocytosis in the maturation of the membrane-associated mucin MUC1. Mol Biol Cell. 1997;8:301a.
-
- Barylko B, Binns D, Lin K-M, Atkinson MAL, Jameson DM, Yin HL, Albanesi JP. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J Biol Chem. 1998;273:3791–3797. - PubMed
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Bierhuizen MFA, Maemura K, Fukuda M. Expression of a differentiation antigen and poly-N-acetyllactosaminyl O-glycans directed by a cloned core 2 β-1,6-N-acetylglucosaminyltransferase. J Biol Chem. 1994;269:4473–4479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

