Transforming growth factor beta(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1)
- PMID: 10712520
- PMCID: PMC14831
- DOI: 10.1091/mbc.11.3.1061
Transforming growth factor beta(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1)
Abstract
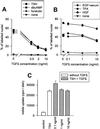
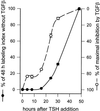
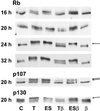
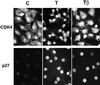
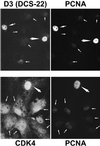
Dog thyroid epithelial cells in primary culture constitute a physiologically relevant model of positive control of DNA synthesis initiation and G0-S prereplicative phase progression by cAMP as a second messenger for thyrotropin (thyroid-stimulating hormone [TSH]). As previously shown in this system, the cAMP-dependent mitogenic pathway differs from growth factor cascades as it stimulates the accumulation of p27(kip1) but not cyclins D. Nevertheless, TSH induces the nuclear translocations and assembly of cyclin D3 and cdk4, which are essential in cAMP-dependent mitogenesis. Here we demonstrate that transforming growth factor beta(1) (TGFbeta(1)) selectively inhibits the cAMP-dependent cell cycle in mid-G1 and various cell cycle regulatory events, but it weakly affects the stimulation of DNA synthesis by epidermal growth factor (EGF), hepatocyte growth factor, serum, and phorbol esters. EGF+serum and TSH did not interfere importantly with TGFbeta receptor signaling, because they did not affect the TGFbeta-induced nuclear translocation of Smad 2 and 3. TGFbeta inhibited the phosphorylation of Rb, p107, and p130 induced by TSH, but it weakly affected the phosphorylation state of Rb-related proteins in EGF+serum-treated cells. TGFbeta did not inhibit c-myc expression. In TSH-stimulated cells, TGFbeta did not affect the expression of cyclin D3, cdk4, and p27(kip1), nor the induced formation of cyclin D3-cdk4 complexes, but it prevented the TSH-induced relocalization of p27(kip1) from cdk2 to cyclin D3-cdk4. It prevented the nuclear translocations of cdk4 and cyclin D3 without altering the assembly of cyclin D3-cdk4 complexes probably formed in the cytoplasm, where they were prevented from sequestering nuclear p27(kip1) away from cdk2. This study dissociates the assembly of cyclin D3-cdk4 complexes from their nuclear localization and association with p27(kip1). It provides a new mechanism of regulation of proliferation by TGFbeta, which points out the subcellular location of cyclin D-cdk4 complexes as a crucial factor integrating mitogenic and antimitogenic regulations in an epithelial cell in primary culture.
Figures








References
-
- Alexandrow MG, Moses HL. Transforming growth factor beta 1 inhibits mouse keratinocytes late in G1 independent of effects on gene transcription. Cancer Res. 1995;55:3928–3932. - PubMed
-
- Baptist M, Dumont JE, Roger PP. Demonstration of cell cycle kinetics in thyroid primary culture by immunostaining of proliferating cell nuclear antigen: differences in cyclic AMP-dependent and -independent mitogenic stimulations. J Cell Sci. 1993;105:69–80. - PubMed
-
- Baptist M, Dumont JE, Roger PP. Intercellular heterogeneity of early mitogenic events: cAMP generalizes the EGF effect on c-Fos protein appearance but not on MAP kinase phosphorylation and nuclear translocation in dog thyroid epithelial cells. Exp Cell Res. 1995;221:160–171. - PubMed
-
- Baptist M, Lamy F, Gannon J, Hunt T, Dumont JE, Roger PP. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J Cell Physiol. 1996;166:256–273. - PubMed
-
- Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990;1032:79–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

