Characterization of the Mycobacterium tuberculosis iniBAC promoter, a promoter that responds to cell wall biosynthesis inhibition
- PMID: 10714983
- PMCID: PMC101861
- DOI: 10.1128/JB.182.7.1802-1811.2000
Characterization of the Mycobacterium tuberculosis iniBAC promoter, a promoter that responds to cell wall biosynthesis inhibition
Abstract
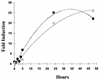
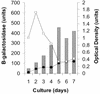
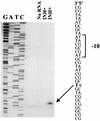
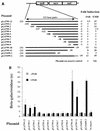
The cell wall provides an attractive target for antibiotics against Mycobacterium tuberculosis. Agents such as isoniazid and ethambutol that work by inhibiting cell wall biosynthesis are among the most highly effective antibiotics against this pathogen. Although considerable progress has been made identifying the targets for cell wall active antibiotics, little is known about the intracellular mechanisms that are activated as a consequence of cell wall injury. These mechanisms are likely to have an important role in growth regulation and in the induction of cell death by antibiotics. We previously discovered three isoniazid-induced genes (iniB, iniA, and iniC) organized in tandem on the M. tuberculosis genome. Here, we investigate the unique features of the putative iniBAC promoter. This promoter was specifically induced by a broad range of inhibitors of cell wall biosynthesis but was not inducible by other conditions that are toxic to mycobacteria via other mechanisms. Induction required inhibitory concentrations of antibiotics and could be detected only in actively growing cells. Analysis of the iniBAC promoter sequence revealed both a regulatory element upstream and a potential repressor binding region downstream of the transcriptional start site. The induction phenotype and structure of the iniBAC promoter suggest that a complex intracellular response occurs when cell wall biosynthesis is inhibited in M. tuberculosis and other mycobacteria.
Figures





References
-
- Ad Hoc Committee of the Scientific Assembly on Microbiology, Tuberculosis, and Pulmonary Infections. Treatment of tuberculosis and tuberculosis infection in adults and children. Clin Infect Dis. 1995;21:9–27.
-
- Alland D, Kalkut G E, Moss A R, McAdam R A, Hahn J A, Bosworth W, Drucker E, Bloom B R. Transmission of tuberculosis in New York City: an analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. - PubMed
-
- Alland D, Kramnik I, Weisbrod T R, Otsubo L, Miller L P, Jacobs W R, Jr, Bloom B R. Identification of differentially expressed mRNA in prokaryotic organisms by customized amplification libraries (DECAL): the effect of isoniazid on gene expression in Mycobacterium tuberculosis. Proc Nat Acad Sci USA. 1998;22:13227–13232. - PMC - PubMed
-
- Allen N E, Hobbs J N. Induction of vancomycin resistance in Enterococcus faecium by non-glycopeptide antibiotics. FEMS Microbiol Lett. 1995;132:107–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

