Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis
- PMID: 10716706
- PMCID: PMC16259
- DOI: 10.1073/pnas.97.7.3444
Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis
Abstract
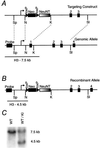
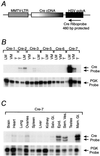
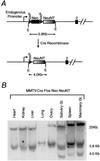
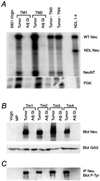
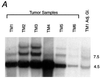
The neu (c-erbB-2, Her-2) protooncogene is amplified and overexpressed in 20-30% of human breast cancers. Although transgenic mouse models have illustrated the role of Neu in the induction of mammary tumors, Neu expression in these models is driven by a strong viral promoter of questionable relevance to the human disease. To ascertain whether expression of activated Neu under the control of the endogenous promoter in the mammary gland could induce mammary tumors we have generated mice that conditionally express activated Neu under the transcriptional control of the intact endogenous Neu promoter. Expression of oncogenic neu in the mammary gland resulted in accelerated lobulo-alveolar development and formation of focal mammary tumors after a long latency period. However, expression of activated Neu under the normal transcriptional control of the endogenous promoter was not sufficient for the initiation of mammary carcinogenesis. Strikingly, all mammary tumors bear amplified copies (2-22 copies) of the activated neu allele relative to the wild-type allele and express highly elevated levels of neu transcript and protein. Thus, like human erbB-2-positive breast tumors, mammary tumorigenesis in this mouse model requires the amplification and commensurate elevated expression of the neu gene.
Figures
References
-
- Dougall W C, Qian X, Peterson N C, Miller M J, Samanta A, Greene M I. Oncogene. 1994;9:2109–2123. - PubMed
-
- Hynes N E, Stern D F. Biochim Biophys Acta. 1994;1198:165–184. - PubMed
-
- Ullrich A, Coussens L, Hayflick J S, Dull T J, Gray A, Tam A W, Lee J, Yarden Y, Libermann T A, Schlessinger J. Nature (London) 1984;309:418–425. - PubMed
-
- Bargmann C I, Hung M C, Weinberg R A. Nature (London) 1986;319:226–230. - PubMed
-
- Coussens L, Yang-Feng T L, Liao Y C, Chen E, Gray A, McGrath J, Seeburg P H, Libermann T A, Schlessinger J, Francke U. Science. 1985;230:1132–1139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

