The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC)
- PMID: 10716723
- PMCID: PMC16312
- DOI: 10.1073/pnas.97.7.3753
The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC)
Abstract
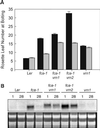
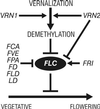
In Arabidopsis, the MADS-box protein encoded by FLOWERING LOCUS C (FLC) is a repressor of flowering. Vernalization, which promotes flowering in the late-flowering ecotypes and many late-flowering mutants, decreases the level of FLC transcript and protein in the plant. This vernalization-induced reduction in FLC transcript levels is mitotically stable and occurs in all tissues. FLC activity is restored in each generation, as is the requirement of a low-temperature exposure for the promotion of flowering. The level of FLC determines the extent of the vernalization response in the promotion of flowering, and there is a quantitative relationship between the duration of cold treatment and the extent of down-regulation of FLC activity. We conclude that FLC is the central regulator of the induction of flowering by vernalization. Other vernalization-responsive late-flowering mutants, which are disrupted in genes that encode regulators of FLC, are late-flowering as a consequence of their elevated levels of FLC.
Figures




References
-
- Koornneef M, Blankestijn-de Vries H, Hanhart C, Soppe W, Peeters T. Plant J. 1994;6:911–919.
-
- Lee I, Michaels S D, Masshardt A S, Amasino R M. Plant J. 1994;6:903–909.
-
- Sanda S, Amasino R M. Weeds World. 1995;2:2–8.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

