Protonation of the beta-lactam nitrogen is the trigger event in the catalytic action of class A beta-lactamases
- PMID: 10716727
- PMCID: PMC16209
- DOI: 10.1073/pnas.97.7.3160
Protonation of the beta-lactam nitrogen is the trigger event in the catalytic action of class A beta-lactamases
Abstract
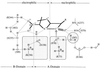
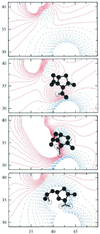
The pH dependence of the pK(a) values of all ionizable groups and of the electrostatic potential at grid points corresponding to catalytically important atoms in the active site of TEM-1 beta-lactamase has been calculated by a mean-field approach for reaction intermediates modeled on the basis of energy minimized x-ray crystallographic coordinates. By estimating electrostatic contributions to the free energy changes accompanying the conversion of the free enzyme into the acylenzyme reaction intermediate, we found that acid-catalyzed protonation of the beta-lactam nitrogen is energetically favored as the initiating event, followed by base-catalyzed nucleophilic attack on the carbonyl carbon of the beta-lactam group. N-protonation is catalyzed through a hydrogen-bonded cluster involving the 2-carboxylate group of the substrate, the side chains of S130 and K234, and a solvent molecule. Nucleophilic attack on the carbonyl carbon is carried out by the side chain of S70 with proton abstraction catalyzed by a water molecule hydrogen-bonded to the side chain of E166. Stabilization of ion pairs in the active site through interactions with distant clusters of charged residues in the enzyme was concluded to be an important driving force of the catalytic mechanism.
Figures


References
-
- Neu H C. Science. 1992;257:1064–1073. - PubMed
-
- Knox J S, Moews P C, Frere J M. Chem Biol. 1996;3:937–947. - PubMed
-
- Strynadka N C J, Adachi H, Jensen S E, Johns K, Sielecki A, Betzel C, Sutoh K, James M N G. Nature (London) 1992;359:700–705. - PubMed
-
- Chen C H H, Rahil J, Pratt R F, Herzberg O. J Mol Biol. 1993;234:165–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

