Oligomerization of serotonin transporter and its functional consequences
- PMID: 10716733
- PMCID: PMC16200
- DOI: 10.1073/pnas.97.7.3106
Oligomerization of serotonin transporter and its functional consequences
Abstract
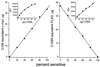
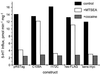
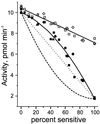
Two forms of serotonin transporter (SERT) were prepared with different epitope tags. When co-expressed in HeLa cells, the form containing a FLAG tag (Res-FLAG) was associated with the form containing a c-myc tag (Sens-myc). Antibody against c-myc precipitated Res-FLAG from detergent extracts of cells expressing both forms, but not when Res-FLAG was expressed alone. The specificity of the interaction was demonstrated by the observation that anti-myc antibodies did not precipitate the unrelated vesicular stomatitis virus coat glycoprotein when it was co-expressed with Sens-myc. Sens-myc contained a reactive cysteine at position 172, which reacted with both (2-aminoethyl)methanethiosulfonate and N-biotinylaminoethyl methanethiosulfonate on the surface of intact cells. Sens-myc, but not Res-FLAG, was inactivated by these reagents. When co-expressed with Sens-myc, functionally active Res-FLAG was precipitated by immobilized streptavidin from digitonin-solubilized cells that had been treated with N-biotinylaminoethyl methanethiosulfonate. In cells co-expressing mixtures of Sens-myc and Res-FLAG, the amount of inactivation by (2-aminoethyl)methanethiosulfonate was less than expected if the two forms were independent. The results are consistent with a dimeric form of SERT with functional interactions between subunits, and with association of dimers into a higher order complex, possibly a tetramer.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

