An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes
- PMID: 10716943
- PMCID: PMC316417
An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes
Abstract
CAD (caspase-activated DNase) can cause DNA fragmentation in apoptotic cells. Transgenic mice that ubiquitously express a caspase-resistant form of the CAD inhibitor (ICAD) were generated. Thymocytes prepared from the mice were resistant to DNA fragmentation induced by a variety of stimuli. However, similar numbers of TUNEL-positive cells were present in adult tissues of transgenic and wild-type mice. Exposure to gamma-irradiation caused a striking increase in the number of TUNEL-positive cells in the thymus of wild-type, but not transgenic, mice. TUNEL-positive nuclei in transgenic mice were confined to thymic macrophages. When apoptotic thymocytes from the transgenic mice were cocultured with macrophages, the thymocytes underwent phagocytosis and their chromosomal DNA underwent fragmentation. This DNA fragmentation was sensitive to inhibitors that block the acidification of lysosomes. Hence, we conclude that the DNA fragmentation that occurs during apoptosis not only can result cell-autonomously from CAD activity but can also be attributed to a lysosomal acid DNase(s), most likely DNase II, after the apoptotic cells are engulfed.
Figures
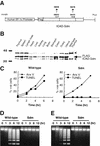



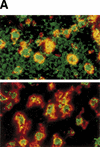


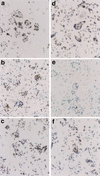

References
-
- Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in the peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. - PubMed
-
- Aliprantis AO, Diez-Roux G, Mulder LC, Zychlinsky A, Lang RA. Do macrophages kill through apoptosis? Immunol Today. 1996;17:573–576. - PubMed
-
- Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. - PubMed
-
- Baker KP, Baron WF, Henzel WJ, Spencer SA. Molecular cloning and characterization of human and murine DNase II. Gene. 1998;215:281–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
