Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis
- PMID: 10716993
- PMCID: PMC15979
- DOI: 10.1073/pnas.97.6.2626
Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis
Abstract
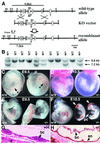
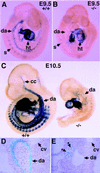
The activin receptor-like kinase 1 (ALK1) is a type I receptor for transforming growth factor-beta (TGF-beta) family proteins. Expression of ALK1 in blood vessels and mutations of the ALK1 gene in human type II hereditary hemorrhagic telangiectasia patients suggest that ALK1 may have an important role during vascular development. To define the function of ALK1 during development, we inactivated the ALK1 gene in mice by gene targeting. The ALK1 homozygous embryos die at midgestation, exhibiting severe vascular abnormalities characterized by excessive fusion of capillary plexes into cavernous vessels and hyperdilation of large vessels. These vascular defects are associated with enhanced expression of angiogenic factors and proteases and are characterized by deficient differentiation and recruitment of vascular smooth muscle cells. The blood vessel defects in ALK1-deficient mice are reminiscent of mice lacking TGF-beta1, TGF-beta type II receptor (TbetaR-II), or endoglin, suggesting that ALK1 may mediate TGF-beta1 signal in endothelial cells. Consistent with this hypothesis, we demonstrate that ALK1 in endothelial cells binds to TGF-beta1 and TbetaR-II. Furthermore, the ALK1 signaling pathway can inhibit TGF-beta1-dependent transcriptional activation mediated by the known TGF-beta1 type I receptor, ALK5. Taken together, our results suggest that the balance between the ALK1 and ALK5 signaling pathways in endothelial cells plays a crucial role in determining vascular endothelial properties during angiogenesis.
Figures



References
-
- Hoodless P A, Wrana J L. Curr Top Microbial Immunol. 1998;228:235–272. - PubMed
-
- Risau W. Nature (London) 1997;386:671–674. - PubMed
-
- Pepper M S. Cytokines Growth Factor Rev. 1997;8:21–43. - PubMed
-
- Gajdusek C M, Luo Z, Mayberg M R. J Cell Physiol. 1993;157:133–144. - PubMed
-
- Pepper M S, Vassalli J-D, Orci L, Montesano R. Exp Cell Res. 1993;204:356–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

