Polyclonal antibodies from patients immunized with a globo H-keyhole limpet hemocyanin vaccine: isolation, quantification, and characterization of immune responses by using totally synthetic immobilized tumor antigens
- PMID: 10716997
- PMCID: PMC15996
- DOI: 10.1073/pnas.97.6.2719
Polyclonal antibodies from patients immunized with a globo H-keyhole limpet hemocyanin vaccine: isolation, quantification, and characterization of immune responses by using totally synthetic immobilized tumor antigens
Abstract
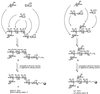
We have previously reported on a carbohydrate-based vaccine program for immunotherapy in cancer patients. One such vaccine, based on the globo H antigen conjugated to the protein keyhole limpet hemocyanin (KLH), has been in clinical evaluation. Although this and other carbohydrate vaccines have been shown to induce antibody responses, there are currently no quantitative data on the antibody levels achieved in immunized patients by these or other anti-cancer vaccines. We report herein an efficient route to complex synthetic oligosaccharides attached to an affinity matrix for identifying and isolating antibodies elicited against such a carbohydrate-based vaccine in humans. Pre- and postvaccination profiles from serum samples of patients immunized with globo H-KLH were compared. All anti-globo H antibody activity was efficiently separated from other serum constituents. The isolated antibodies were readily quantified, and their specificities were analyzed. Since no comparable data were available on antibodies resulting from the vaccination of other cancer patients, we compared the observed levels with those quoted in studies with bacterial polysaccharide vaccines that had been quantified. Remarkably, cancer patients immunized with globo H-KLH produce anti-globo H antibody levels often exceeding those formed by immunization with bacterial polysaccharides. In addition, substantial quantities of both IgG and IgM antibodies were elicited, clearly indicating a class switch to IgG. Taken together, these analyses serve to clarify several aspects of the immune response to the vaccine and give several new insights to the carbohydrate-based vaccination strategy. Furthermore, antibodies so isolated could well have applications in clinical therapy.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

