Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington's disease
- PMID: 10717003
- PMCID: PMC16027
- DOI: 10.1073/pnas.97.6.2898
Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington's disease
Abstract
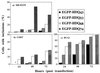
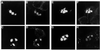
Huntington's disease (HD), spinocerebellar ataxias types 1 and 3 (SCA1, SCA3), and spinobulbar muscular atrophy (SBMA) are caused by CAG/polyglutamine expansion mutations. A feature of these diseases is ubiquitinated intraneuronal inclusions derived from the mutant proteins, which colocalize with heat shock proteins (HSPs) in SCA1 and SBMA and proteasomal components in SCA1, SCA3, and SBMA. Previous studies suggested that HSPs might protect against inclusion formation, because overexpression of HDJ-2/HSDJ (a human HSP40 homologue) reduced ataxin-1 (SCA1) and androgen receptor (SBMA) aggregate formation in HeLa cells. We investigated these phenomena by transiently transfecting part of huntingtin exon 1 in COS-7, PC12, and SH-SY5Y cells. Inclusion formation was not seen with constructs expressing 23 glutamines but was repeat length and time dependent for mutant constructs with 43-74 repeats. HSP70, HSP40, the 20S proteasome and ubiquitin colocalized with inclusions. Treatment with heat shock and lactacystin, a proteasome inhibitor, increased the proportion of mutant huntingtin exon 1-expressing cells with inclusions. Thus, inclusion formation may be enhanced in polyglutamine diseases, if the pathological process results in proteasome inhibition or a heat-shock response. Overexpression of HDJ-2/HSDJ did not modify inclusion formation in PC12 and SH-SY5Y cells but increased inclusion formation in COS-7 cells. To our knowledge, this is the first report of an HSP increasing aggregation of an abnormally folded protein in mammalian cells and expands the current understanding of the roles of HDJ-2/HSDJ in protein folding.
Figures



References
-
- Perutz M F. Trend Biochem Sci. 1999;24:58–63. - PubMed
-
- DiFiglia M, Sapp E, Chase K O, Davies S W, Bates G P, Vonsattel J P, Aronin N. Science. 1997;277:1990–1993. - PubMed
-
- Li M, Miwa S, Kobayashi Y, Merry D E, Yamamoto M, Tanaka F, Doyu M, Hashizume Y, Fischbeck K H, Sobue G. Ann Neurol. 1998;44:249–254. - PubMed
-
- Igarashi S, Koide R, Shimohata T, Yamada M, Hayashi Y, Takano H, Date H, Oyake M, Sato T, Sato A, et al. Nat Genet. 1998;18:111–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

