Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses
- PMID: 10717006
- PMCID: PMC16032
- DOI: 10.1073/pnas.97.6.2928
Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses
Abstract
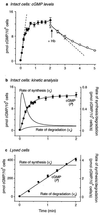
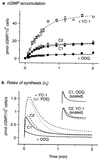
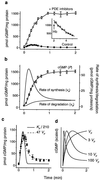
A major receptor for nitric oxide (NO) is the cGMP-synthesizing enzyme, soluble guanylyl cyclase (sGC), but it is not known how this enzyme behaves in cells. In cerebellar cells, NO (from diethylamine NONOate) increased astrocytic cGMP with a potency (EC(50) </= 20 nM) higher than that reported for purified sGC. Deactivation of NO-stimulated sGC activity, studied by trapping free NO with hemoglobin, took place within seconds (or less) rather than the minute time scale reported for the purified enzyme. Measurement of the rates of accumulation and degradation of cGMP were used to follow the activity of sGC over time. The peak activity, occurring within seconds of adding NO, was swiftly followed by desensitization to a steady-state level 8-fold lower. The same desensitizing profile was observed when the net sGC activity was increased or decreased or when cGMP breakdown was inhibited. Recovery from desensitization was relatively slow (half-time = 1.5 min). When the cells were lysed, sGC desensitization was lost. Analysis of the transient cGMP response to NO in human platelets showed that sGC underwent a similar desensitization. The results indicate that, in its natural environment, sGC behaves much more like a neurotransmitter receptor than had been expected from previous enzymological studies, and that hitherto unknown sGC regulatory factors exist. Rapid sGC desensitization, in concert with variations in the rate of cGMP breakdown, provides a fundamental mechanism for shaping cellular cGMP responses and is likely to be important in decoding NO signals under physiological and pathophysiological conditions.
Figures



References
-
- Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. - PubMed
-
- Garthwaite J, Boulton C L. Annu Rev Physiol. 1995;57:683–706. - PubMed
-
- Waldman S A, Murad F. Pharmacol Rev. 1987;39:163–196. - PubMed
-
- Ignarro L J. Biochem Pharmacol. 1991;41:485–490. - PubMed
-
- Sunahara R K, Dessauer C W, Gilman A G. Annu Rev Pharmacol Toxicol. 1996;36:461–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

