Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants
- PMID: 10717008
- PMCID: PMC16034
- DOI: 10.1073/pnas.97.6.2940
Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants
Abstract
Despite the recognition of H(2)O(2) as a central signaling molecule in stress and wounding responses, pathogen defense, and regulation of cell cycle and cell death, little is known about how the H(2)O(2) signal is perceived and transduced in plant cells. We report here that H(2)O(2) is a potent activator of mitogen-activated protein kinases (MAPKs) in Arabidopsis leaf cells. Using epitope tagging and a protoplast transient expression assay, we show that H(2)O(2) can activate a specific Arabidopsis mitogen-activated protein kinase kinase kinase, ANP1, which initiates a phosphorylation cascade involving two stress MAPKs, AtMPK3 and AtMPK6. Constitutively active ANP1 mimics the H(2)O(2) effect and initiates the MAPK cascade that induces specific stress-responsive genes, but it blocks the action of auxin, a plant mitogen and growth hormone. The latter observation provides a molecular link between oxidative stress and auxin signal transduction. Finally, we show that transgenic tobacco plants that express a constitutively active tobacco ANP1 orthologue, NPK1, display enhanced tolerance to multiple environmental stress conditions without activating previously described drought, cold, and abscisic acid signaling pathways. Thus, manipulation of key regulators of an oxidative stress signaling pathway, such as ANP1/NPK1, provides a strategy for engineering multiple stress tolerance that may greatly benefit agriculture.
Figures
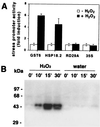

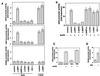
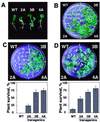
Comment in
-
Connecting oxidative stress, auxin, and cell cycle regulation through a plant mitogen-activated protein kinase pathway.Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2405-7. doi: 10.1073/pnas.97.6.2405. Proc Natl Acad Sci U S A. 2000. PMID: 10716978 Free PMC article. No abstract available.
-
Plant mitogen-activated protein kinase cascades: Negative regulatory roles turn out positive.Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):784-6. doi: 10.1073/pnas.98.3.784. Proc Natl Acad Sci U S A. 2001. PMID: 11158543 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

