Non-linear membrane properties of sacral sphincter motoneurones in the decerebrate cat
- PMID: 10718752
- PMCID: PMC2269836
- DOI: 10.1111/j.1469-7793.2000.00741.x
Non-linear membrane properties of sacral sphincter motoneurones in the decerebrate cat
Abstract
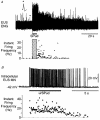
1. Responses to pudendal afferent stimulation and depolarizing intracellular current injection were examined in sacral sphincter motoneurones in decerebrate cats. 2. In 16 animals examined, 2-10 s trains of electrical stimulation of pudendal afferents evoked sustained sphincter motoneurone activity lasting from 5 to >50 s after stimulation. The sustained response was observed in: 11 animals in the absence of any drugs; two animals after the intravenous administration of 5-hydroxytryptophan (5-HTP; <= 20 mg kg-1); one animal in which methoxamine was perfused onto the ventral surface of the exposed spinal cord; and two animals following the administration of intravenous noradrenergic agonists. 3. Extracellular and intracellular recordings from sphincter motoneurones revealed that the persistent firing evoked by afferent stimulation could be terminated by motoneurone membrane hyperpolarization during micturition or by intracellular current injection. 4. Intracellular recordings revealed that 22/40 sphincter motoneurones examined displayed a non-linear, steep increase in the membrane potential in response to depolarizing ramp current injection. The mean voltage threshold for this non-linear membrane response was -43 +/- 3 mV. Five of the 22 cells displaying the non-linear membrane response were recorded prior to the administration of 5-HTP; 17 after the intravenous administration of 5-HTP (<= 20 mg kg-1). 5. It is concluded that sphincter motoneurones have a voltage-sensitive, non-linear membrane response to depolarization that could contribute to sustained sphincter motoneurone firing during continence.
Figures






References
-
- Beattie MS, Li Q, Leedy MG, Bresnahan JC. Motoneurons innervating the external anal and urethral sphincter of the female cat have different patterns of dendritic arborisation. Neuroscience Letters. 1990;111:69–74. - PubMed
-
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hind limb motoneurones of decerebrate cats. Journal of Neurophysiology. 1998a;80:2023–2037. - PubMed
-
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hind limb motoneurones of decerebrate cat. Journal of Neurophysiology. 1998b;80:2038–2045. - PubMed
-
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, Smith J, editors. Handbook of Physiology, Neural Control of Movement. Vol. 1. Oxford: Oxford University Press; 1996. pp. 3–53. assoc.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

