Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina
- PMID: 10718754
- PMCID: PMC2269821
- DOI: 10.1111/j.1469-7793.2000.t01-1-00771.x
Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina
Abstract
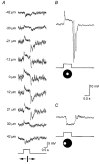
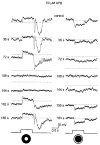
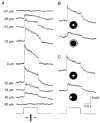
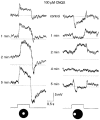
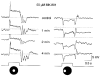
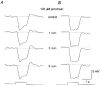
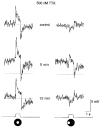
1. Intracellular recordings were obtained from neurons in the superfused retina-eyecup preparation of the rabbit under dark-adapted conditions. Neurotransmitter agonists and antagonists were applied exogenously via the superfusate to dissect the synaptic pathways pharmacologically and thereby determine those pathways responsible for the generation of the on-centre/off-surround receptive fields of AII amacrine cells. 2. Application of the metabotropic glutamate receptor agonist, APB, reversibly blocked both the on-centre and off-surround responses of AII cells. These data were consistent with the idea that both the centre- and surround-mediated responses are derived from inputs from the presynaptic rod bipolar cells. 3. Whereas rod bipolar cells showed on-receptive fields approximately 100 microm across, we found no evidence for an antagonistic off-surround response using light stimuli which effectively elicited the off-surrounds of AII amacrine cells. These results indicated that the surrounds of AII cells are not derived from rod bipolar cell inputs. 4. Application of the ionotropic glutamate receptor antagonists CNQX or DNQX enhanced the on-centre responses of AII cells but attenuated the off-surround responses. These data indicated that the centre- and surround-mediated responses could not both be derived from signals crossing the rod bipolar-to-AII cell synapse. 5. Application of the glycine antagonist, strychnine, had only minor and variable effects on AII cell responses. However, the GABA antagonists picrotoxin and bicuculline enhanced the on-centre response but attenuated or completely blocked the off-surround response of AII cells. The GABA antagonists had no effect on the responses of horizontal cells indicating that their effects on AII cell responses reflected actions on inner retinal circuitry rather than feedback circuitry in the outer plexiform layer. 6. Application of the voltage-gated sodium channel blocker TTX enhanced the on-centre responses of AII cells but attenuated or abolished their off-surround responses. 7. Taken together, our results suggest that the on-centre responses of AII cells result from the major excitatory drive from rod bipolar cells. However, the surround receptive fields of AII cells appear to be generated by lateral, inhibitory signals derived from neighbouring GABAergic, on-centre amacrine cells. A model is presented whereby the S1 amacrine cells produce the surround receptive fields of AII amacrine cells via inhibitory, feedback circuitry to the axon terminals of rod bipolar cells.
Figures
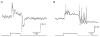


References
-
- Bloomfield SA. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. Journal of Neurophysiology. 1992;68:711–725. - PubMed
-
- Bloomfield SA. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. Journal of Neurophysiology. 1996;75:1878–1893. - PubMed
-
- Bloomfield SA, Dowling JE. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. Journal of Neurophysiology. 1985a;53:699–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

