Ischaemic changes in refractoriness of human cutaneous afferents under threshold-clamp conditions
- PMID: 10718757
- PMCID: PMC2269819
- DOI: 10.1111/j.1469-7793.2000.t01-1-00807.x
Ischaemic changes in refractoriness of human cutaneous afferents under threshold-clamp conditions
Abstract
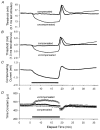
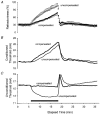
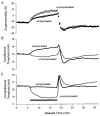
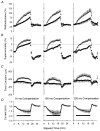
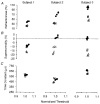
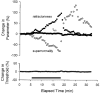
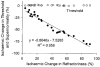
1. A technique was developed to counteract the changes in threshold to electrical stimuli of large myelinated cutaneous afferents in the human median nerve induced by ischaemia for 13 min. Intermittent application of polarizing currents was used in five subjects, in whom refractoriness, supernormality and the strength-duration time constant (tauSD) were tracked to determine whether compensating for the ischaemia-induced changes in threshold also controlled the ischaemic changes in these excitability parameters. 2. The threshold compensation prevented the ischaemic changes in tauSD, an excitability parameter dependent on nodal Na+ channels. Threshold compensation did not prevent the changes in refractoriness and supernormality, whether the compensation began 10, 100 or 200 ms prior to the test stimuli. 3. In three subjects, continuous polarizing current was injected for 13 min to compensate for the ischaemic change in threshold, thus clamping threshold at the pre-ischaemic level. Again, tauSD was effectively controlled, but there were still ischaemic changes in refractoriness and supernormality. 4. The effective control of tauSD suggests that both the intermittent threshold compensation and the continuous threshold clamp effectively controlled membrane potential at the node of Ranvier. 5. The ischaemic increase in refractoriness when threshold was kept constant could be due to interference with the processes responsible for refractoriness by a metabolic product of ischaemia. The ischaemic change in supernormality during effective compensation probably results from the intrusion of refractoriness into the conditioning-test intervals normally associated with maximal supernormality. 6. The present results indicate that ischaemia has effects on axonal excitability that cannot be readily explained by changes in membrane potential. Specifically, it is suggested that ischaemic metabolites interfere with the recovery of Na+ channels from inactivation.
Figures
References
-
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. Journal of Neurophysiology. 1997;77:1503–1513. - PubMed
-
- Baker MD, Bostock H. Inactivation of macroscopic late Na+ current and characteristics of unitary late Na+ currents in sensory neurons. Journal of Neurophysiology. 1998;80:2538–2549. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

