Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export
- PMID: 10722314
- PMCID: PMC305578
- DOI: 10.1093/emboj/19.3.410
Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export
Abstract
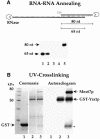
Mex67p and Mtr2p constitute an essential mRNA export complex that interacts with poly(A)+ RNA and nuclear pore proteins. We have identified Yra1p, an intranuclear protein with in vitro RNA-RNA annealing activity, which directly binds to Mex67p. The complex between Yra1p and Mex67p was reconstituted in vitro and shown by UV-crosslinking to bind directly to RNA. Mutants of YRA1 are impaired in nuclear poly(A)+ RNA export at restrictive growth conditions. ALY, the mouse homologue of Yra1p and a transcriptional coactivator, can bind in vitro to yeast and human Mex67p and partly complements the otherwise non-viable yra1 null mutant. Thus, Yra1p is the first RNA-binding protein characterized, which bridges the shuttling Mex67p/Mtr2p exporter to intranuclear mRNA transport cargoes.
Figures


References
-
- Braspenning J., Manetti, R., Zumbach, K., Meschede, W., Gissmann, L. and Tommasino, M. (1997) A general purification protocol for e7 proteins from ‘high- and low-risk’ human papillomavirus types expressed in the yeast Schizosaccharomyces pombe. Protein Expr. Purif., 10, 192–201. - PubMed
-
- Bruhn L., Munnerlyn, A. and Grosschedl, R. (1997) ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRα enhancer function. Genes Dev., 11, 640–653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

