Pefloxacin-induced achilles tendon toxicity in rodents: biochemical changes in proteoglycan synthesis and oxidative damage to collagen
- PMID: 10722483
- PMCID: PMC89784
- DOI: 10.1128/AAC.44.4.867-872.2000
Pefloxacin-induced achilles tendon toxicity in rodents: biochemical changes in proteoglycan synthesis and oxidative damage to collagen
Abstract
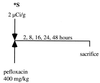
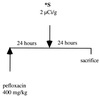
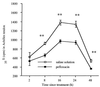
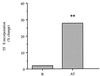
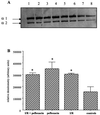
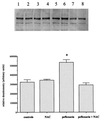
Despite a relatively low incidence of serious side effects, fluoroquinolones and the fluoroquinolone pefloxacin have been reported to occasionally promote tendinopathy that might result in the complication of spontaneous rupture of tendons. In the present study, we investigated in rodents the intrinsic deleterious effect of pefloxacin (400 mg/kg of body weight) on Achilles tendon proteoglycans and collagen. Proteoglycan synthesis was determined by measurement of in vivo and ex vivo radiosulfate incorporation in mice. Collagen oxidative modifications were measured by carbonyl derivative detection by Western blotting. An experimental model of tendinous ischemia (2 h) and reperfusion (3 days) was achieved in rats. Biphasic changes in proteoglycan synthesis were observed after a single administration of pefloxacin, consisting of an early inhibition followed by a repair-like phase. The depletion phase was accompanied by a marked decrease in the endogenous serum sulfate level and a concomitant increase in the level of sulfate excretion in urine. Studies of ex vivo proteoglycan synthesis confirmed the in vivo results that were obtained. The decrease in proteoglycan anabolism seemed to be a direct effect of pefloxacin on tissue metabolism rather than a consequence of the low concentration of sulfate. Pefloxacin treatment for several days induced oxidative damage of type I collagen, with the alterations being identical to those observed in the experimental tendinous ischemia and reperfusion model. Oxidative damage was prevented by coadministration of N-acetylcysteine (150 mg/kg) to the mice. These results provide the first experimental evidence of a pefloxacin-induced oxidative stress in the Achilles tendon that altered proteoglycan anabolism and oxidized collagen.
Figures

References
-
- Birch H L, Rutter G A, Goodship A E. Oxidative energy metabolism in equine tendon cells. Res Vet Sci. 1997;62:93–97. - PubMed
-
- Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radical Biol Med. 1997;22:515–524. - PubMed
-
- Burkhardt J E, Hill M A, Carlton W W, Kesterson J W. Histologic and histochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1990;27:162–170. - PubMed
-
- Christ W, Lehnert T, Ullbrich B. Specific toxicologic aspects of the quinolones. Rev Infect Dis. 1988;10(Suppl.):S144–S146. - PubMed
-
- Desbordes J M, Bouquet S, Breux J, Chapelle G, Bataille B, Becq Giraudon B, Fusciardi J. Comparative penetration of 3 fluoroquinolones into human cranial bone tissue after administration of a single intravenous dose. Drugs. 1993;45(Suppl. 3):448–449.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

