A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1
- PMID: 10722492
- PMCID: PMC89793
- DOI: 10.1128/AAC.44.4.920-928.2000
A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1
Abstract
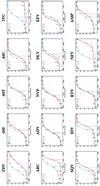
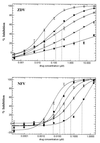
Although combination antiretroviral therapy has resulted in a considerable improvement in the treatment of human immunodeficiency virus (HIV) type 1 (HIV-1) infection, the emergence of resistant virus is a significant obstacle to the effective management of HIV infection and AIDS. We have developed a novel phenotypic drug susceptibility assay that may be useful in guiding therapy and improving long-term suppression of HIV replication. Susceptibility to protease (PR) and reverse transcriptase (RT) inhibitors is measured by using resistance test vectors (RTVs) that contain a luciferase indicator gene and PR and RT sequences derived from HIV-1 in patient plasma. Cells are transfected with RTV DNA, resulting in the production of virus particles that are used to infect target cells. Since RTVs are replication defective, luciferase activity is measured following a single round of replication. The assay has been automated to increase throughput and is completed in 8 to 10 days. Test results may be useful in facilitating the selection of optimal treatment regimens for patients who have failed prior therapy or drug-naive patients infected with drug-resistant virus. In addition, the assay can be used to evaluate candidate drugs and assist in the development of new drugs that are active against resistant strains of HIV-1.
Figures

References
-
- Ausubel F, editor. Current protocols in molecular biology. 1 and 2. New York, N.Y: John Wiley & Sons, Inc.; 1999.
-
- Boden D, Hurley A, Zhang L, Cao Y, Guo Y, Duran M, Tsay J, Ip J, Farthing C, Limoli K, Parkin N, Markowitz M. HIV-1 drug resistance in newly infected individuals. JAMA. 1999;282:1135–1141. - PubMed
-
- Byrnes V W, Emini E A, Schleif W A, Condra J H, Schneider C L, Long W J, Wolfgang J A, Graham D J, Gotlib L, Schlabach A J, Wolanski B S, Blahy O M, Quintero J C, Rhodes A, Rothy E, Titus D L, Sardana V V. Susceptibilities of human immunodeficiency virus type 1 enzyme and viral variants expressing multiple resistance-engendering amino acid substitutions to reverse transcriptase inhibitors. Antimicrob Agents Chemother. 1994;38:1404–1407. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

