Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria
- PMID: 10722499
- PMCID: PMC89800
- DOI: 10.1128/AAC.44.4.972-977.2000
Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria
Abstract
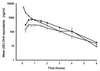
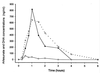
The pharmacokinetic properties of oral and intravenous artesunate (2 mg/kg of body weight) were studied in 19 adult patients with acute uncomplicated Plasmodium falciparum malaria by using a randomized crossover design. A sensitive bioassay was used to measure the antimalarial activity in plasma which results from artesunate and its principal metabolite, dihydroartemisinin. The oral study was repeated with 15 patients during convalescence. The mean absolute oral bioavailability of the antimalarial agent in patients with acute malaria was 61% (95% confidence interval [CI], 52 to 70%). The absorption and elimination of oral artesunate were rapid, with a mean elimination half-life of antimalarial activity of 43 min (95% CI, 33 to 53 min). Following oral administration to patients with acute falciparum malaria, peak antimalarial activity in plasma and the area under the plasma concentration-time curve were approximately double those during convalescence and the apparent volume of distribution and clearance were approximately half those during convalescence (P < or = 0.005). Acute malaria is associated with a significant reduction in the clearance of artesunate-associated antimalarial activity.
Figures
References
-
- Ashton M, Hai T N, Sy N D, Huong D X, Huong N V, Nieu N T, Cong L D. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos. 1998;26:25–27. - PubMed
-
- Barradell L B, Fitton A. Artesunate: a review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. - PubMed
-
- Batty K T, Thu L T A, Illett K F, Mai T X, Hung N C, Tien N P, Powell S M, Thien H V, Binh T Q, Kim N V, Davis T M E. A pharmacokinetic and pharmacodynamic study of artesunate in vivax malaria. Am J Trop Med Hyg. 1998;59:823–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

