Single-dose pharmacokinetics and safety of the oral antiviral compound adefovir dipivoxil in children infected with human immunodeficiency virus type 1. The Pediatrics AIDS Clinical Trials Group
- PMID: 10722509
- PMCID: PMC89810
- DOI: 10.1128/AAC.44.4.1041-1046.2000
Single-dose pharmacokinetics and safety of the oral antiviral compound adefovir dipivoxil in children infected with human immunodeficiency virus type 1. The Pediatrics AIDS Clinical Trials Group
Abstract
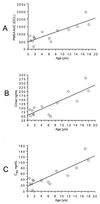
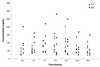
The acyclic phosphonate analog adefovir is a potent inhibitor of retroviruses, including human immunodeficiency virus (HIV) type 1, and, unlike some antiviral nucleosides, does not require the initial phosphorylation step for its activity. Two oral dosages of the adefovir prodrug adefovir dipivoxil were evaluated in a phase I study with children with HIV infection. A total of 14 patients were stratified into age groups ranging from 6 months to 18 years of age. Eight patients received 1.5 mg of adefovir dipivoxil per kg of body weight, and six patients received 3.0 mg of adefovir dipivoxil per kg. Serum samples were obtained at intervals during the 8 h postdosing and were analyzed for adefovir concentrations. Patients were monitored for adverse effects. All samples collected resulted in quantifiable levels of adefovir (lower limit of quantitation, 25 ng/ml) from each patient. The areas under the concentration-versus-time curves (AUCs) were similar (P = 0.85) for the 1.5- and 3.0-mg/kg doses, while the apparent oral clearance (CL/F) was significantly higher (P = 0.05) for the 3-mg/kg dose. Pharmacokinetic parameters differed by patient age. In comparing those children older and younger than the median age of 5.1 years, AUC (P = 0.03), maximum concentration of drug in serum (P = 0.004), and the concentration at 8 h postdosing (P = 0.02) were significantly lower for the younger children. There were no significant differences for apparent volume of distribution and CL/F normalized to body surface area, but there was a suggestive difference in half-life (P = 0.07) among the subjects in the older and younger age groups. No significant adverse events were encountered. These data provide the basis for a multidose phase II study of adefovir dipivoxil in HIV-infected infants and children.
Figures


References
-
- Balzarini J, et al. Potent anti-simian immunodeficiency virus (SIV) activity and pharmacokinetics of 9-(2-phosphonylmethoxyethyl) adenine (PMEA) in rhesus monkeys. In: Schellekens H, Horzinek M C, editors. Animal models in AIDS. New York, N.Y: Elsevier Science Publications; 1990. pp. 131–138.
-
- Balzarini J, Naesens L, Slachmuylders J, Niphuis H, Rosenberg L, Holy A, Schellekens H, DeClercq E. 9-(2-Phosphonylmethoxyethyl) adenine (PMEA) effectively inhibits retrovirus replication in vivo and Simian immunodeficiency virus (SIV) infection in rhesus monkeys. AIDS. 1991;5:21–28. - PubMed
-
- Barditch-Crovo P, Toole J, Hendrix C W, Cundy K C, Ebling D, Jaffe H S, Lietman P S. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxil (9-[2-(bis-pivaloyloxymethyl)-phosphonylmethoxyethyl]adenine) in HIV-infected patients. J Infect Dis. 1997;176:406–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

