Potent and selective activity of a combination of thymidine and 1843U89, a folate-based thymidylate synthase inhibitor, against Plasmodium falciparum
- PMID: 10722510
- PMCID: PMC89811
- DOI: 10.1128/AAC.44.4.1047-1050.2000
Potent and selective activity of a combination of thymidine and 1843U89, a folate-based thymidylate synthase inhibitor, against Plasmodium falciparum
Abstract
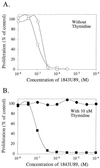
Unlike mammalian cells, malarial parasites are completely dependent on the de novo pyrimidine pathway and lack the enzymes to salvage preformed pyrimidines. In the present study, first, it is shown that 1843U89, even without polyglutamylation, is a potent folate-based inhibitor of purified malarial parasite thymidylate synthase. The binding was noncompetitive with respect to methylenetetrahydrofolate, and 1843U89 had a K(i) of 1 nM. The compound also had potent antimalarial activity in vitro. Plasmodium falciparum cells in culture were inhibited by 1843U89, with a 50% inhibitory concentration of about 70 nM. The compound was effective against drug-sensitive as well as drug-resistant clones of P. falciparum. As predicted by the biochemistry of the parasite, the potent inhibition of parasite proliferation by 1843U89 could not be reversed with 10 microM thymidine. In contrast, in the presence of 10 microM thymidine, mammalian cells were unaffected by 1843U89 even at concentrations as high as 0.1 mM, thus offering a selectivity window of more than 1,000-fold. On this basis, folate-based thymidylate synthase inhibitors may represent a powerful additional tool that can be used to combat drug-resistant malaria.
Figures


Similar articles
-
Susceptibility of Plasmodium falciparum to a combination of thymidine and ICI D1694, a quinazoline antifolate directed at thymidylate synthase.Antimicrob Agents Chemother. 1994 Mar;38(3):476-80. doi: 10.1128/AAC.38.3.476. Antimicrob Agents Chemother. 1994. PMID: 8203840 Free PMC article.
-
Biochemical and cellular pharmacology of 1843U89, a novel benzoquinazoline inhibitor of thymidylate synthase.Cancer Res. 1993 Feb 15;53(4):810-8. Cancer Res. 1993. PMID: 8428362
-
Destruction of WiDr multicellular tumor spheroids with the novel thymidylate synthase inhibitor 1843U89 at physiological thymidine concentrations.Cancer Chemother Pharmacol. 1994;33(6):455-9. doi: 10.1007/BF00686500. Cancer Chemother Pharmacol. 1994. PMID: 8137455
-
Folate-based thymidylate synthase inhibitors as anticancer drugs.Ann Oncol. 1995 Nov;6(9):871-81. doi: 10.1093/oxfordjournals.annonc.a059353. Ann Oncol. 1995. PMID: 8624289 Review.
-
Antifolates in clinical development.Semin Oncol. 1997 Oct;24(5 Suppl 18):S18-40-S18-51. Semin Oncol. 1997. PMID: 9420020 Review.
Cited by
-
Recent highlights in antimalarial drug resistance and chemotherapy research.Trends Parasitol. 2008 Dec;24(12):537-44. doi: 10.1016/j.pt.2008.09.005. Epub 2008 Oct 18. Trends Parasitol. 2008. PMID: 18938106 Free PMC article. Review.
-
Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice.J Med Chem. 2009 Apr 9;52(7):1864-72. doi: 10.1021/jm801343r. J Med Chem. 2009. PMID: 19296651 Free PMC article.
-
Catalytic mechanism of α-phosphate attack in dUTPase is revealed by X-ray crystallographic snapshots of distinct intermediates, 31P-NMR spectroscopy and reaction path modelling.Nucleic Acids Res. 2013 Dec;41(22):10542-55. doi: 10.1093/nar/gkt756. Epub 2013 Aug 27. Nucleic Acids Res. 2013. PMID: 23982515 Free PMC article.
-
Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential.J Med Chem. 2011 Aug 11;54(15):5540-61. doi: 10.1021/jm200592f. Epub 2011 Jul 14. J Med Chem. 2011. PMID: 21696174 Free PMC article.
-
Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum.J Med Chem. 2008 Jun 26;51(12):3649-53. doi: 10.1021/jm8001026. Epub 2008 Jun 4. J Med Chem. 2008. PMID: 18522386 Free PMC article.
References
-
- Carreras C W, Santi D V. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem. 1995;64:721–762. - PubMed
-
- Chen G X, Zolg J W. Purification of the bifunctional thymidylate synthase-dihydrofolate reductase complex from the human malaria parasite Plasmodium falciparum. Mol Pharmacol. 1987;32:723–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

