Role of toxin functional domains in anthrax pathogenesis
- PMID: 10722564
- PMCID: PMC97348
- DOI: 10.1128/IAI.68.4.1781-1786.2000
Role of toxin functional domains in anthrax pathogenesis
Abstract
We investigated the role of the functional domains of anthrax toxins during infection. Three proteins produced by Bacillus anthracis, the protective antigen (PA), the lethal factor (LF), and the edema factor (EF), combine in pairs to produce the lethal (PA+LF) and edema (PA+EF) toxins. A genetic strategy was developed to introduce by allelic exchange specific point mutations or in-frame deletions into B. anthracis toxin genes, thereby impairing either LF metalloprotease or EF adenylate cyclase activity or PA functional domains. In vivo effects of toxin mutations were analyzed in an experimental infection of mice. A tight correlation was observed between the properties of anthrax toxins delivered in vivo and their in vitro activities. The synergic effects of the lethal and edema toxins resulted purely from their enzymatic activities, suggesting that in vivo these toxins may act together. The PA-dependent antibody response to LF induced by immunization with live B. anthracis was used to follow the in vivo interaction of LF and PA. We found that the binding of LF to PA in vivo was necessary and sufficient for a strong antibody response against LF, whereas neither LF activity nor binding of lethal toxin complex to the cell surface was required. Mutant PA proteins were cleaved in mice sera. Thus, our data provide evidence that, during anthrax infection, PA may interact with LF before binding to the cell receptor. Immunoprotection studies indicated that the strain producing detoxified LF and EF, isogenic to the current live vaccine Sterne strain, is a safe candidate for use as a vaccine against anthrax.
Figures
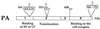


References
-
- Bragg T S, Robertson D L. Nucleotide sequence and analysis of the lethal factor gene (lef) from Bacillus anthracis. Gene. 1986;81:45–54. - PubMed
-
- Brossier F, Guidi-Rontani C, Mock M. Anthrax toxins. C R Soc Biol. 1998;192:437–444. - PubMed
-
- Duesbery N S, Webb C P, Leppla S H, Gordon V M, Klimpel K R, Copeland T D, Ahn N G, Oskarsson M K, Fukasawa K, Paull K D, Vande woude G F. Proteolytic inactivation of map-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

