Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon
- PMID: 10722593
- PMCID: PMC97377
- DOI: 10.1128/IAI.68.4.1988-1996.2000
Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon
Abstract
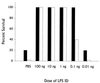
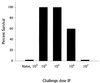
Previous results have demonstrated that nonspecific protective immunity against lethal Francisella tularensis live vaccine strain (LVS) or Listeria monocytogenes infection can be stimulated either by sublethal infection with bacteria or by treatment with bacterial DNA given 3 days before lethal challenge. Here we characterize the ability of purified lipopolysaccharide (LPS) from F. tularensis LVS to stimulate similar early protective immunity. Treatment of mice with surprisingly small amounts of LVS LPS resulted in very strong and long-lived protection against lethal LVS challenge within 2 to 3 days. Despite this strong protective response, LPS purified from F. tularensis LVS did not activate murine B cells for proliferation or polyclonal immunoglobulin secretion, nor did it activate murine splenocytes for secretion of interleukin-4 (IL-4), IL-6, IL-12, or gamma interferon (IFN-gamma). Immunization of mice with purified LVS LPS induced a weak specific anti-LPS immunoglobulin M (IgM) response and very little IgG; however, infection of mice with LVS bacteria resulted in vigorous IgM and IgG, particularly IgG2a, anti-LPS antibody responses. Studies using various immunodeficient mouse strains, including LPS-hyporesponsive C3H/HeJ mice, muMT(-) (B-cell-deficient) knockout mice, and IFN-gamma-deficient mice, demonstrated that the mechanism of protection does not involve recognition through the Lps(n) gene product; nonetheless, protection was dependent on B cells as well as IFN-gamma.
Figures


Similar articles
-
Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis.Microbes Infect. 2003 Apr;5(5):397-403. doi: 10.1016/s1286-4579(03)00052-2. Microbes Infect. 2003. PMID: 12737995
-
Interleukin-17 protects against the Francisella tularensis live vaccine strain but not against a virulent F. tularensis type A strain.Infect Immun. 2013 Sep;81(9):3099-105. doi: 10.1128/IAI.00203-13. Epub 2013 Jun 17. Infect Immun. 2013. PMID: 23774604 Free PMC article.
-
Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge.Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4343-8. doi: 10.1073/pnas.0813411106. Epub 2009 Feb 26. Proc Natl Acad Sci U S A. 2009. PMID: 19251656 Free PMC article.
-
Mucosal immunopathogenesis of Francisella tularensis.Ann N Y Acad Sci. 2007 Jun;1105:266-83. doi: 10.1196/annals.1409.007. Epub 2007 Mar 29. Ann N Y Acad Sci. 2007. PMID: 17395728 Review.
-
The structure and function of Francisella lipopolysaccharide.Ann N Y Acad Sci. 2007 Jun;1105:202-18. doi: 10.1196/annals.1409.006. Epub 2007 Mar 29. Ann N Y Acad Sci. 2007. PMID: 17395723 Free PMC article. Review.
Cited by
-
Novel catanionic surfactant vesicle vaccines protect against Francisella tularensis LVS and confer significant partial protection against F. tularensis Schu S4 strain.Clin Vaccine Immunol. 2014 Feb;21(2):212-26. doi: 10.1128/CVI.00738-13. Epub 2013 Dec 18. Clin Vaccine Immunol. 2014. PMID: 24351755 Free PMC article.
-
The role of B cells and humoral immunity in Mycobacterium tuberculosis infection.Semin Immunol. 2014 Dec;26(6):588-600. doi: 10.1016/j.smim.2014.10.005. Epub 2014 Oct 28. Semin Immunol. 2014. PMID: 25458990 Free PMC article. Review.
-
Tularemia vaccines.Folia Microbiol (Praha). 2016 Nov;61(6):495-504. doi: 10.1007/s12223-016-0461-z. Epub 2016 May 19. Folia Microbiol (Praha). 2016. PMID: 27194547 Review.
-
Effective, broad spectrum control of virulent bacterial infections using cationic DNA liposome complexes combined with bacterial antigens.PLoS Pathog. 2010 May 27;6(5):e1000921. doi: 10.1371/journal.ppat.1000921. PLoS Pathog. 2010. PMID: 20523903 Free PMC article.
-
Protection afforded by heat shock protein 60 from Francisella tularensis is due to copurified lipopolysaccharide.Infect Immun. 2004 Jul;72(7):4109-13. doi: 10.1128/IAI.72.7.4109-4113.2004. Infect Immun. 2004. PMID: 15213156 Free PMC article.
References
-
- Anthony L S D, Kongshavn P A L. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987;2:3–14. - PubMed
-
- Anthony L S D, Morrissey P J, Nano F E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from l-arginine metabolism. J Immunol. 1992;148:1829–1834. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

