Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response
- PMID: 10722598
- PMCID: PMC97382
- DOI: 10.1128/IAI.68.4.2024-2033.2000
Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response
Abstract
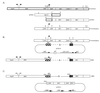
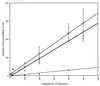
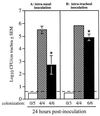
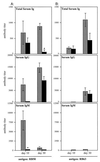
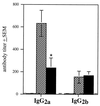
Fimbriae are filamentous, cell surface structures which have been proposed to mediate attachment of Bordetella species to respiratory epithelium. Bordetella bronchiseptica has four known fimbrial genes: fim2, fim3, fimX, and fimA. While these genes are unlinked on the chromosome, their protein products are assembled and secreted by a single apparatus encoded by the fimBCD locus. The fimBCD locus is embedded within the fha operon, whose genes encode another putative adhesin, filamentous hemagglutinin (FHA). We have constructed a Fim(-) B. bronchiseptica strain, RB63, by introducing an in-frame deletion extending from fimB through fimD. Western blot analysis showed that RB63 is unable to synthesize fimbriae but is unaffected for FHA expression. Using this mutant, we assessed the role of fimbriae in pathogenesis in vitro and in vivo in natural animal hosts. Although RB63 was not significantly defective in its ability to adhere to various tissue culture cell lines, including human laryngeal HEp-2 cells, it was considerably altered in its ability to cause respiratory tract infections in rats. The number of DeltafimBCD bacteria recovered from the rat trachea at 10 days postinoculation was significantly decreased compared to that of wild-type B. bronchiseptica and was below the limit of detection at 30 and 60 days postinoculation. The number of bacteria recovered from the nasal cavity and larynx was not significantly different between RB63 and the wild-type strain at any time point. The ability of fimbriae to mediate initial attachment to tracheal tissue was tested in an intratracheal inoculation assay. Significantly fewer RB63 than wild-type bacteria were recovered from the tracheas at 24 h after intratracheal inoculation. These results demonstrate that fimbriae are involved in enhancing the ability of B. bronchiseptica to establish tracheal colonization and are essential for persistent colonization at this site. Interestingly, anti-Bordetella serum immunoglobulin M (IgM) levels were significantly lower in animals infected with RB63 than in animals infected with wild-type B. bronchiseptica at 10 days postinoculation. Even at 30 days postinoculation, RB63-infected animals had lower serum anti-Bordetella antibody titers in general. This disparity in antibody profiles suggests that fimbriae are also important for the induction of a humoral immune response.
Figures

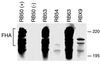

References
-
- Abraham S N, Jonsson A B, Normark S. Fimbriae-mediated host-pathogen cross-talk. Curr Opin Microbiol. 1998;1:75–81. - PubMed
-
- Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. - PubMed
-
- Asakura K, Saito H, Hata M, Kataura A. Antigen specific immunoglobulin production by NALT lymphocytes in rats. Acta Oto-Laryngol Suppl. 1996;523:80–83. - PubMed
-
- Binder J, Graser E, Hancock W W, Wasowska B, Sayegh M H, Volk H D, Kupiec-Weglinski J W. Downregulation of intragraft IFN-gamma expression correlates with increased IgG1 alloantibody response following intrathymic immunomodulation of sensitized rat recipients. Transplantation. 1995;60:1516–1524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

