Nonopsonic binding of type III Group B Streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway
- PMID: 10722601
- PMCID: PMC97385
- DOI: 10.1128/IAI.68.4.2053-2060.2000
Nonopsonic binding of type III Group B Streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway
Abstract
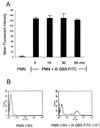
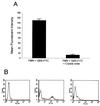
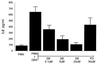
Nonopsonic interaction of host immune cells with pathogens is an important first line of defense. We hypothesized that nonopsonic recognition between type III group B streptococcus and human neutrophils would occur and that the interaction would be sufficient to trigger neutrophil activation. By using a serum-free system, it was found that heat-killed type III group B streptococci bound to neutrophils in a rapid, stable, and inoculum-dependent manner that did not result in ingestion. Transposon-derived type III strain COH1-13, which lacks capsular polysaccharide, and strain COH1-11 with capsular polysaccharide lacking terminal sialic acid demonstrated increased neutrophil binding, suggesting that capsular polysaccharide masks an underlying binding site. Experiments using monoclonal antibodies to complement receptor 1 and to the I domain or lectin site of complement receptor 3 did not inhibit binding, indicating that the complement receptors used for ingestion of opsonized group B streptococci were not required for nonopsonic binding. Nonopsonic binding resulted in rapid activation of cellular p38 and p44/42 mitogen-activated protein kinases. This interaction was not an effective trigger for superoxide production but did promote release of the proinflammatory cytokine interleukin-8. The release of interleukin-8 was markedly suppressed by the p38 mitogen-activated protein kinase inhibitor SB203580 but was only minimally suppressed by the mitogen-activated protein/extracellular signal-regulated kinase inhibitor PD98059. Thus, nonopsonic binding of type III group B streptococci to neutrophils is sufficient to initiate intracellular signaling pathways and could serve as an arm of innate immunity of particular importance to the immature host.
Figures




References
-
- Baker, C. J., and M. S. Edwards. Group B streptococcal infections. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed., in press. The W. B. Saunders Co., Philadelphia, Pa.
-
- Cantinieaux B, Hariga C, Courtoy J, Hupin J, Fondu P. Staphylococcus aureus phagocytosis. A new cytofluorometric method using FITC and paraformaldehyde. J Immunol Methods. 1989;121:203–208. - PubMed
-
- Cohen M S. Molecular events in the activation of human neutrophils for microbial killing. Clin Infect Dis. 1994;18(Suppl. 2):S170–S179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

