Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers
- PMID: 10722611
- PMCID: PMC97395
- DOI: 10.1128/IAI.68.4.2135-2141.2000
Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers
Abstract
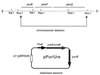
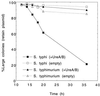
Attenuated Salmonella enterica serovar Typhi has been studied as an oral vaccine vector. Despite success with attenuated S. enterica serovar Typhimurium vectors in animals, early clinical trials of S. enterica serovar Typhi expressing heterologous antigens have shown that few subjects have detectable immune responses to vectored antigens. A previous clinical study of phoP/phoQ-deleted S. enterica serovar Typhi expressing Helicobacter pylori urease from a multicopy plasmid showed that none of eight subjects had detectable immune responses to the vectored antigen. In an attempt to further define the variables important for engendering immune responses to vectored antigens in humans, six volunteers were inoculated with 5 x 10(7) to 8 x 10(7) CFU of phoP/phoQ-deleted S. enterica serovar Typhimurium expressing the same antigen. Two of the six volunteers had fever; none had diarrhea, bacteremia, or other serious side effects. The volunteers were more durably colonized than in previous studies of phoP/phoQ-deleted S. enterica serovar Typhi. Five of the six volunteers seroconverted to S. enterica serovar Typhimurium antigens and had strong evidence of anti-Salmonella mucosal immune responses by enzyme-linked immunospot studies. Three of six (three of five who seroconverted to Salmonella) had immune responses in the most sensitive assay of urease-specific immunoglobulin production by blood mononuclear cells in vitro. One of these had a fourfold or greater increase in end-point immunoglobulin titer in serum versus urease. Attenuated S. enterica serovar Typhimurium appears to be more effective than S. enterica serovar Typhi for engendering immune responses to urease. Data suggest that this may be related to a greater stability of antigen-expressing plasmid in S. enterica serovar Typhimurium and/or prolonged intestinal colonization. Specific factors unique to nontyphoidal salmonellae may also be important for stimulation of the gastrointestinal immune system.
Figures

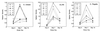
References
-
- Buchwald D, Blaser M. A review of human salmonellosis. II. Duration of excretion following infection with nontyphi Salmonella. Rev Infect Dis. 1984;6:345–346. - PubMed
-
- Carlstedt G, Dahl P, Niklasson P M, Gullberg K, Banck G, Kahlmeter G. Norfloxacin treatment of salmonellosis does not shorten the carrier state. Scand J Infect Dis. 1990;22:553–556. - PubMed
-
- Corthesy-Theulaz I, Hopkins S, Bachmann D, Saldinger P, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bousourene H, Blum A L, Kraehenbuhl J P. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium expressing urease A and B subunits. Infect Immun. 1998;66:581–586. - PMC - PubMed
-
- DiPetrillo M D, Tibbetts T, Kleanthous H, Killeen K P, Hohmann E L. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine. 1999;18:449–459. - PubMed
-
- Forrest B D. Identification of an intestinal immune response using peripheral blood lymphocytes. Lancet. 1988;i:81–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

