Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2
- PMID: 10722617
- PMCID: PMC97401
- DOI: 10.1128/IAI.68.4.2171-2182.2000
Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2
Abstract
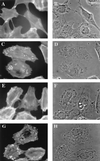
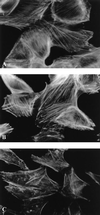
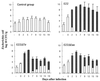
Attaching and effacing (A/E) rabbit enteropathogenic Escherichia coli (REPEC) strains belonging to serogroup O103 are an important cause of diarrhea in weaned rabbits. Like human EPEC strains, they possess the locus of enterocyte effacement clustering the genes involved in the formation of the A/E lesions. In addition, pathogenic REPEC O103 strains produce an Esp-dependent but Eae (intimin)-independent alteration of the host cell cytoskeleton characterized by the formation of focal adhesion complexes and the reorganization of the actin cytoskeleton into bundles of stress fibers. To investigate the role of intimin and its translocated coreceptor (Tir) in the pathogenicity of REPEC, we have used a newly constructed isogenic tir null mutant together with a previously described eae null mutant. When human HeLa epithelial cells were infected, the tir mutant was still able to induce the formation of stress fibers as previously reported for the eae null mutant. When the rabbit epithelial cell line RK13 was used, REPEC O103 produced a classical fluorescent actin staining (FAS) effect, whereas both the eae and tir mutants were FAS negative. In a rabbit ligated ileal loop model, neither mutant was able to induce A/E lesions. In contrast to the parental strain, which intimately adhered to the enterocytes and destroyed the brush border microvilli, bacteria of both mutants were clustered in the mucus without reaching and damaging the microvilli. The role of intimin and Tir was then analyzed in vivo by oral inoculation of weaned rabbits. Although both mutants were still present in the intestinal flora of the rabbits 3 weeks after oral inoculation, neither mutant strain induced any clinical signs or significant weight loss in the inoculated rabbits whereas the parental strain caused the death of 90% of the inoculated rabbits. Nevertheless, an inflammatory infiltrate was present in the lamina propria of the rabbits infected with both mutants, with an inflammatory response greater for the eae null mutant. In conclusion, we have confirmed the role of intimin in virulence, and we have shown, for the first time, that Tir is also a key factor in vivo for pathogenicity.
Figures





References
-
- Adams L M, Simmons C P, Rezmann L, Strugnell R A, Robins-Browne R M. Identification and characterization of a K88- and CS31A-like operon of a rabbit enteropathogenic Escherichia coli strain which encodes fimbriae involved in the colonization of rabbit intestine. Infect Immun. 1997;65:5222–5230. - PMC - PubMed
-
- An H, Fairbrother J M, Dubreuil J D, Harel J. Cloning and characterization of the eae gene from a dog attaching and effacing Escherichia coli strain 4221. FEMS Microbiol Lett. 1997;148:239–245. - PubMed
-
- Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

