Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection
- PMID: 10722619
- PMCID: PMC97403
- DOI: 10.1128/IAI.68.4.2187-2195.2000
Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection
Erratum in
- Infect Immun 2000 Sep;68(9):5469
Abstract
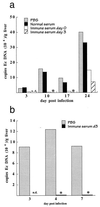
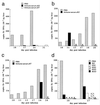
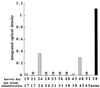
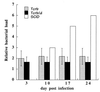
It is generally accepted that cellular, but not humoral immunity, plays an important role in host defense against intracellular bacteria. However, studies of some of these pathogens have provided evidence that antibodies can provide immunity if present during the initiation of infection. Here, we examined immunity against infection by Ehrlichia chaffeensis, an obligate intracellular bacterium that causes human monocytic ehrlichiosis. Studies with mice have demonstrated that immunocompetent strains are resistant to persistent infection but that SCID mice become persistently and fatally infected. Transfer of immune serum or antibodies obtained from immunocompetent C57BL/6 mice to C57BL/6 scid mice provided significant although transient protection from infection. Bacterial clearance was observed when administration occurred at the time of inoculation or well after infection was established. The effect was dose dependent, occurred within 2 days, and persisted for as long as 2 weeks. Weekly serum administration prolonged the survival of susceptible mice. Although cellular immunity is required for complete bacterial clearance, the data show that antibodies can play a significant role in the elimination of this obligate intracellular bacterium during active infection and thus challenge the paradigm that humoral responses are unimportant for immunity to such organisms.
Figures


References
-
- Acharya I L, Lowe C U, Thapa R, Gurubacharya V L, Shrestha M B, Cadoz M, Schulz D, Armand J, Bryla D A, Birger Trollfors M P H, Cramton T, Schneerson R, Robbins J B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. N Engl J Med. 1987;317:1101–1104. - PubMed
-
- Alarcón-Segovia D, Ruiz-Argüelles A, Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163–164. - PubMed
-
- Allen J E, Maizels R M. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. - PubMed
-
- Brieland J K, Heath L A, Huffnagle G B, Remick D G, McClain M S, Hurley M C, Kunkel R K, Fantone J C, Engleberg C. Humoral immunity and regulation of intrapulmonary growth of Legionella pneumophila in the immunocompetent host. J Immunol. 1996;157:5002–5008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

