The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection
- PMID: 10722630
- PMCID: PMC97414
- DOI: 10.1128/IAI.68.4.2276-2285.2000
The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection
Abstract
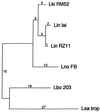
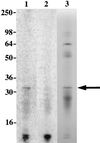
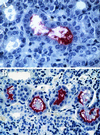
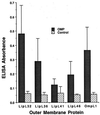
We report the cloning of the gene encoding the 32-kDa lipoprotein, designated LipL32, the most prominent protein in the leptospiral protein profile. We obtained the N-terminal amino acid sequence of a staphylococcal V8 proteolytic-digest fragment to design an oligonucleotide probe. A Lambda-Zap II library containing EcoRI fragments of Leptospira kirschneri DNA was screened, and a 5.0-kb DNA fragment which contained the entire structural lipL32 gene was identified. Several lines of evidence indicate that LipL32 is lipid modified in a manner similar to that of other procaryotic lipoproteins. The deduced amino acid sequence of LipL32 would encode a 272-amino-acid polypeptide with a 19-amino-acid signal peptide, followed by a lipoprotein signal peptidase cleavage site. LipL32 is intrinsically labeled during incubation of L. kirschneri in media containing [(3)H]palmitate. The linkage of palmitate and the amino-terminal cysteine of LipL32 is acid labile. LipL32 is completely solubilized by Triton X-114 extraction of L. kirschneri; phase separation results in partitioning of LipL32 exclusively into the hydrophobic, detergent phase, indicating that it is a component of the leptospiral outer membrane. CaCl(2) (20 mM) must be present during phase separation for recovery of LipL32. LipL32 is expressed not only during cultivation but also during mammalian infection. Immunohistochemistry demonstrated intense LipL32 reactivity with L. kirschneri infecting proximal tubules of hamster kidneys. LipL32 is also a prominent immunogen during human leptospirosis. The sequence and expression of LipL32 is highly conserved among pathogenic Leptospira species. These findings indicate that LipL32 may be important in the pathogenesis, diagnosis, and prevention of leptospirosis.
Figures


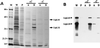
References
-
- Bergstrom S, Bundoc V G, Barbour A G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. - PubMed
-
- Bolin C A, Cassells J A, Zuerner R L, Trueba G. Effect of vaccination with a monovalent Leptospira interrogans serovar hardjo type hardjo-bovis vaccine on type hardjo-bovis infection of cattle. Am J Vet Res. 1991;52:1639–1643. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

