Vasorelaxant and antiplatelet activity of 4,7-dimethyl-1,2, 5-oxadiazolo[3,4-d]pyridazine 1,5,6-trioxide: role of soluble guanylate cyclase, nitric oxide and thiols
- PMID: 10725265
- PMCID: PMC1571938
- DOI: 10.1038/sj.bjp.0703156
Vasorelaxant and antiplatelet activity of 4,7-dimethyl-1,2, 5-oxadiazolo[3,4-d]pyridazine 1,5,6-trioxide: role of soluble guanylate cyclase, nitric oxide and thiols
Abstract
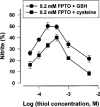
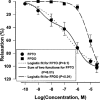
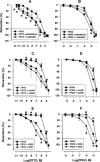
1. Certain heterocyclic N-oxides are vasodilators and inhibitors of platelet aggregation. The pharmacological activity of the furoxan derivative condensed with pyridazine di-N-oxide 4,7-dimethyl-1,2, 5-oxadiazolo[3,4-d]pyridazine 1,5,6-trioxide (FPTO) and the corresponding furazan (FPDO) was studied. 2. FPTO reacted with thiols generating nitrite (NO), S-nitrosoglutathione and hydroxylamine (nitroxyl) and converted oxyHb to metHb. FPDO did not generate detectable amounts of NO-like species but reacted with thiols and oxyHb. 3. FPTO and FPDO haem-dependently stimulated the activity of soluble guanylate cyclase (sGC) and this stimulation was inhibited by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and by 0.1 mM dithiothreitol. 4. FPTO relaxed noradrenaline-precontracted aortic rings and its concentration-response curve was biphasic (pIC(50)=9. 03+/-0.13 and 5.85+/-0.06). FPDO was significantly less potent vasodilator (pIC(50)=5.19+/-0.14). The vasorelaxant activity of FPTO and FPDO was inhibited by ODQ. oxyHb significantly inhibited only FPTO-dependent relaxation. 5. FPTO and FPDO were equipotent inhibitors of ADP-induced platelet aggregation (IC(50)=0.63+/-0.15 and 0.49+/-0. 05 microM, respectively). The antiplatelet activity of FPTO (but not FPDO) was partially suppressed by oxyHb. The antiaggregatory effects of FPTO and FPDO were only partially blocked by sGC inhibitors. 6. FPTO and FPDO (10 - 20 microM) significantly increased cyclic GMP levels in aortic rings and platelets and this increase was blocked by ODQ. 7. Thus, FPTO can generate NO and, like FPDO, reacts with thiols and haem. The vasorelaxant activity of FPTO and FPDO is sGC-dependent and a predominant role is played by NO at FPTO concentrations below 1 microM. On the contrary, inhibition of platelet aggregation is only partially related to sGC activation.
Figures
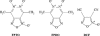


Similar articles
-
The relaxant activity of 4,7-dimethyl-1,2,5-oxadiazolo[3,4-d]-pyridazine 1,5,6-trioxide in the mouse corpus cavernosum.J Pharmacol Exp Ther. 2006 Feb;316(2):753-61. doi: 10.1124/jpet.105.094250. Epub 2005 Oct 27. J Pharmacol Exp Ther. 2006. PMID: 16254132
-
Benzodifuroxan as an NO-dependent activator of soluble guanylate cyclase and a novel highly effective inhibitor of platelet aggregation.Biochemistry (Mosc). 2000 Apr;65(4):457-62. Biochemistry (Mosc). 2000. PMID: 10810184
-
Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity.Br J Pharmacol. 1999 May;127(1):195-203. doi: 10.1038/sj.bjp.0702495. Br J Pharmacol. 1999. PMID: 10369473 Free PMC article.
-
Regulation and role of guanylate cyclase-cyclic GMP in vascular relaxation.Prog Clin Biol Res. 1987;249:65-76. Prog Clin Biol Res. 1987. PMID: 2890172 Review.
-
Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling.Pharmacol Toxicol. 1990 Jul;67(1):1-7. doi: 10.1111/j.1600-0773.1990.tb00772.x. Pharmacol Toxicol. 1990. PMID: 1975691 Review.
Cited by
-
Synthesis and Preliminary Evaluation of N-Oxide Derivatives for the Prevention of Atherothrombotic Events.Molecules. 2015 Oct 7;20(10):18185-200. doi: 10.3390/molecules201018185. Molecules. 2015. PMID: 26457696 Free PMC article.
-
Development and validation of a high-throughput cell-based screen to identify activators of a bacterial two-component signal transduction system.Antimicrob Agents Chemother. 2015 Jul;59(7):3789-99. doi: 10.1128/AAC.00236-15. Epub 2015 Apr 13. Antimicrob Agents Chemother. 2015. PMID: 25870061 Free PMC article.
-
Regulation and Pharmacology of the Cyclic GMP and Nitric Oxide Pathway in Embryonic and Adult Stem Cells.Cells. 2024 Dec 5;13(23):2008. doi: 10.3390/cells13232008. Cells. 2024. PMID: 39682756 Free PMC article. Review.
-
Platelet aggregation is affected by nitrosothiols in patients with chronic hepatitis: in vivo and in vitro studies.World J Gastroenterol. 2007 Jul 21;13(27):3677-83. doi: 10.3748/wjg.v13.i27.3677. World J Gastroenterol. 2007. PMID: 17659726 Free PMC article.
-
Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors.Molecules. 2021 Sep 21;26(18):5705. doi: 10.3390/molecules26185705. Molecules. 2021. PMID: 34577175 Free PMC article. Review.
References
-
- ARNELLE D.A., STAMLER J.S. NO+, NO•, and NO− donation by S-nitrosothiols: Implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. - PubMed
-
- ARNELLE D.R., STAMLER J.S.Detection of hydroxylamine Methods in Nitric Oxide Research 1996John Wiley & Sons Ltd; 541–554.ed. Feelisch, M. & Stamler, J.S. pp
-
- BRZEZINSKI B., ZUNDEL G. Formation of disulfide bonds in the reaction of SH group-containing amino acids with trimethylamine N-oxide. A regulatory mechanism in proteins. FEBS Lett. 1993;333:331–333. - PubMed
-
- CALVINO R., FRUTTERO R., GHIGO D., BOSIA A., PESCARMONA G.P., GASCO A. 4-Methyl-3-(arylsulfonyl)furoxans: a new class of potent inhibitors of platelet aggregation. J. Med. Chem. 1992;35:3296–3300. - PubMed
-
- CHANDRASHEKAR T.K., KRISHNAN V. Optical and magnetic resonance studies of the interaction of metallo tetraphenylporphyrins with nitrobenzofuroxan. Can. J. Chem. 1983;62:475–480.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

