Subcellular compartmentalization of E2F family members is required for maintenance of the postmitotic state in terminally differentiated muscle
- PMID: 10725332
- PMCID: PMC2174298
- DOI: 10.1083/jcb.148.6.1187
Subcellular compartmentalization of E2F family members is required for maintenance of the postmitotic state in terminally differentiated muscle
Abstract
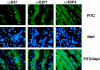
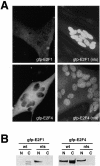
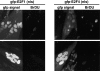
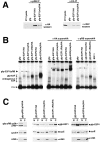
Maintenance of cells in a quiescent state after terminal differentiation occurs through a number of mechanisms that regulate the activity of the E2F family of transcription factors. We report here that changes in the subcellular compartmentalization of the E2F family proteins are required to prevent nuclei in terminally differentiated skeletal muscle from reentering S phase. In terminally differentiated L6 myotubes, E2F-1, E2F-3, and E2F-5 were primarily cytoplasmic, E2F-2 was nuclear, whereas E2F-4 became partitioned between both compartments. In these same cells, pRB family members, pRB, p107, and p130 were also nuclear. This compartmentalization of the E2F-1 and E2F-4 in differentiated muscle cells grown in vitro reflected their observed subcellular location in situ. We determined further that exogenous E2F-1 or E2F-4 expressed in myotubes at levels fourfold greater than endogenous proteins compartmentalized identically to their endogenous counterparts. Only when overexpressed at higher levels was inappropriate subcellular location for these proteins observed. At these levels, induction of the E2F-regulated genes, cyclins A and E, and suppression of factors associated with myogenesis, myogenin, and p21(Cip1) was observed. Only at these levels of E2F expression did nuclei in these terminally differentiated cells enter S phase. These data demonstrate that regulation of the subcellular compartmentalization of E2F-family members is required to maintain nuclei in a quiescent state in terminally differentiated cells.
Figures






Similar articles
-
The E2F-family proteins induce distinct cell cycle regulatory factors in p16-arrested, U343 astrocytoma cells.Oncogene. 1998 Aug 20;17(7):867-76. doi: 10.1038/sj.onc.1202008. Oncogene. 1998. PMID: 9780003
-
Expression and activity of the retinoblastoma protein (pRB)-family proteins, p107 and p130, during L6 myoblast differentiation.Cell Growth Differ. 1995 Oct;6(10):1287-98. Cell Growth Differ. 1995. PMID: 8845306
-
E2F activity is regulated by cell cycle-dependent changes in subcellular localization.Mol Cell Biol. 1997 Dec;17(12):7268-82. doi: 10.1128/MCB.17.12.7268. Mol Cell Biol. 1997. PMID: 9372959 Free PMC article.
-
Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation.Crit Rev Biochem Mol Biol. 1996 Jun;31(3):237-71. doi: 10.3109/10409239609106585. Crit Rev Biochem Mol Biol. 1996. PMID: 8817077 Review.
-
Upregulation of E2F transcription factors in chemically induced mouse skin tumors.Int J Oncol. 1999 Aug;15(2):387-90. doi: 10.3892/ijo.15.2.387. Int J Oncol. 1999. PMID: 10402252 Review.
Cited by
-
E2F4 transcription factor is a prognostic biomarker related to immune infiltration of head and neck squamous cell carcinoma.Sci Rep. 2022 Jul 15;12(1):12132. doi: 10.1038/s41598-022-16541-4. Sci Rep. 2022. PMID: 35840663 Free PMC article.
-
Targeted gene mutation of E2F1 evokes age-dependent synaptic disruption and behavioral deficits.J Neurochem. 2014 Jun;129(5):850-63. doi: 10.1111/jnc.12655. Epub 2014 Feb 12. J Neurochem. 2014. PMID: 24460902 Free PMC article.
-
A non canonical subtilase attenuates the transcriptional activation of defence responses in Arabidopsis thaliana.Elife. 2016 Sep 29;5:e19755. doi: 10.7554/eLife.19755. Elife. 2016. PMID: 27685353 Free PMC article.
-
MyoD synergizes with the E-protein HEB beta to induce myogenic differentiation.Mol Cell Biol. 2006 Aug;26(15):5771-83. doi: 10.1128/MCB.02404-05. Mol Cell Biol. 2006. PMID: 16847330 Free PMC article.
-
Nucleocytoplasmic shuttling of p130/RBL2: novel regulatory mechanism.Mol Cell Biol. 2002 Jan;22(2):453-68. doi: 10.1128/MCB.22.2.453-468.2002. Mol Cell Biol. 2002. PMID: 11756542 Free PMC article.
References
-
- Aguzzi A., Kiess M., Rüedi D., Hamel P.A. Cyclins D1, D2 and D3 are expressed in distinct tissues during mouse embryogenesis. Transgenics. 1996;2:29–39.
-
- Arenzana-Seisdedos F., Turpin P., Rodriguez M., Thomas D., Hay R.T., Virelizier J.L., Dargemont C. Nuclear localization of I kappa B alpha promotes active transport of NF-kappa B from the nucleus to the cytoplasm. J. Cell Sci. 1997;110:369–378. - PubMed
-
- Bandara L.R., La Thangue N.B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351:494–497. - PubMed
-
- Beijersbergen R.L., Kerkhoven R.M., Zhu L., Carlee L., Voorhoeve P.M., Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. - PubMed
-
- Beijersbergen R.L., Carlee L., Kerkhoven R.M., Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

