Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building
- PMID: 10725338
- PMCID: PMC2174314
- DOI: 10.1083/jcb.148.6.1255
Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building
Abstract
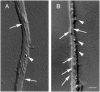
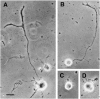
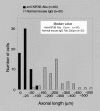
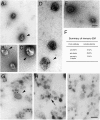
Kinesin superfamily proteins (KIFs) comprise several dozen molecular motor proteins. The KIF3 heterotrimer complex is one of the most abundantly and ubiquitously expressed KIFs in mammalian cells. To unveil the functions of KIF3, microinjection of function-blocking monovalent antibodies against KIF3 into cultured superior cervical ganglion (SCG) neurons was carried out. They significantly blocked fast axonal transport and brought about inhibition of neurite extension. A yeast two-hybrid binding assay revealed the association of fodrin with the KIF3 motor through KAP3. This was further confirmed by using vesicles collected from large bundles of axons (cauda equina), from which membranous vesicles could be prepared in pure preparations. Both immunoprecipitation and immunoelectron microscopy indicated the colocalization of fodrin and KIF3 on the same vesicles, the results reinforcing the evidence that the cargo of the KIF3 motor consists of fodrin-associating vesicles. In addition, pulse-labeling study implied partial comigration of both molecules as fast flow components. Taken together, the KIF3 motor is engaged in fast axonal transport that conveys membranous components important for neurite extension.
Figures





Similar articles
-
The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors.Cell. 1995 Jun 2;81(5):769-80. doi: 10.1016/0092-8674(95)90538-3. Cell. 1995. PMID: 7539720
-
KIF2 is a new microtubule-based anterograde motor that transports membranous organelles distinct from those carried by kinesin heavy chain or KIF3A/B.J Cell Biol. 1995 Apr;129(1):157-67. doi: 10.1083/jcb.129.1.157. J Cell Biol. 1995. PMID: 7535303 Free PMC article.
-
KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport.J Cell Biol. 1995 Sep;130(6):1387-99. doi: 10.1083/jcb.130.6.1387. J Cell Biol. 1995. PMID: 7559760 Free PMC article.
-
The mechanisms of fast and slow transport in neurons: identification and characterization of the new kinesin superfamily motors.Curr Opin Neurobiol. 1997 Oct;7(5):605-14. doi: 10.1016/s0959-4388(97)80079-7. Curr Opin Neurobiol. 1997. PMID: 9384541 Review.
-
Stirring up development with the heterotrimeric kinesin KIF3.Traffic. 2000 Jan;1(1):29-34. doi: 10.1034/j.1600-0854.2000.010105.x. Traffic. 2000. PMID: 11208056 Review.
Cited by
-
Anterograde Axonal Transport in Neuronal Homeostasis and Disease.Front Mol Neurosci. 2020 Sep 18;13:556175. doi: 10.3389/fnmol.2020.556175. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33071754 Free PMC article. Review.
-
A dual role for βII-spectrin in axons.Proc Natl Acad Sci U S A. 2019 Jul 30;116(31):15324-15326. doi: 10.1073/pnas.1909789116. Epub 2019 Jul 9. Proc Natl Acad Sci U S A. 2019. PMID: 31289232 Free PMC article. No abstract available.
-
The axonal transport of mitochondria.J Cell Sci. 2005 Dec 1;118(Pt 23):5411-9. doi: 10.1242/jcs.02745. J Cell Sci. 2005. PMID: 16306220 Free PMC article. Review.
-
The translocation selectivity of the kinesins that mediate neuronal organelle transport.Traffic. 2012 Apr;13(4):549-64. doi: 10.1111/j.1600-0854.2011.01325.x. Epub 2012 Jan 24. Traffic. 2012. PMID: 22212743 Free PMC article.
-
N-acetyl-D-glucosamine kinase interacts with dynein light-chain roadblock type 1 at Golgi outposts in neuronal dendritic branch points.Exp Mol Med. 2015 Aug 14;47(8):e177. doi: 10.1038/emm.2015.48. Exp Mol Med. 2015. PMID: 26272270 Free PMC article.
References
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1997. Current Protocols in Molecular Biology. John Wiley & Sons, Inc. New York. 13.6.2–13.6.5.
-
- Bamburg J.R., Bray D., Chapman K. Assembly of microtubules at the tip of growing axons. Nature. 1986;321:788–790. - PubMed
-
- Beech P.L., Pagh-Roehl K., Noda Y., Hirokawa N., Burnside B., Rosenbaum J.L. Localization of kinesin superfamily proteins to the connecting cilium of fish photoreceptors. J. Cell Sci. 1996;109:889–897. - PubMed
-
- Brady S.T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. - PubMed

