Activation of the human estrogen receptor by the antiestrogens ICI 182,780 and tamoxifen in yeast genetic systems: implications for their mechanism of action
- PMID: 10725345
- PMCID: PMC16302
- DOI: 10.1073/pnas.97.7.3696
Activation of the human estrogen receptor by the antiestrogens ICI 182,780 and tamoxifen in yeast genetic systems: implications for their mechanism of action
Abstract
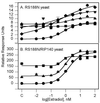
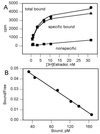
The antiestrogens tamoxifen and ICI 182,780 have been portrayed as competitive antagonists of the estrogen binding site of the alpha-form of the human estrogen receptor (ER). However, in functional studies, neither compound has consistently been able to block estradiol-induced transcription. In this report, three yeast genetic systems were used to investigate the effects of tamoxifen and ICI 182,780 on ER dimerization, transcriptional activation, and the interaction of the receptor with a coactivator, RIP140. Tamoxifen and ICI 182,780 were able to induce ER dimerization and ER-dependent transcription, albeit at up to 15,000-fold higher concentrations than that of estradiol. In the presence of RIP140, the transcription response maximum was increased up to 30-fold for estradiol and both antiestrogens. Whole yeast cell [(3)H]estradiol binding studies demonstrated that tamoxifen could displace the estradiol from the ER, whereas ICI 182,780 treatment resulted in a 4-fold increase in [(3)H]estradiol binding to the receptor. No antagonism of estradiol was observed with tamoxifen or ICI 182,780 in any of the yeast models employed. We have concluded that the antiestrogen activity of compounds like tamoxifen and ICI 182,780 is not caused by their ability to competitively antagonize estradiol binding to the hormone binding site, but possibly by their ability to induce ER-dependent transcription, which in mammalian systems would result in receptor down-regulation. Compounds such as tamoxifen act through the hormone binding site, whereas ICI 182,780 may cause receptor activation through an allosteric binding site.
Figures


Similar articles
-
Yeast two-hybrid system demonstrates that estrogen receptor dimerization is ligand-dependent in vivo.J Biol Chem. 1995 Oct 6;270(40):23322-9. doi: 10.1074/jbc.270.40.23322. J Biol Chem. 1995. PMID: 7559488
-
Allosteric silencing of activating function 1 in the 4-hydroxytamoxifen estrogen receptor complex is induced by substituting glycine for aspartate at amino acid 351.Cancer Res. 2000 Sep 15;60(18):5097-105. Cancer Res. 2000. PMID: 11016635
-
Human estrogen receptor ligand activity inversion mutants: receptors that interpret antiestrogens as estrogens and estrogens as antiestrogens and discriminate among different antiestrogens.Mol Endocrinol. 1996 Mar;10(3):230-42. doi: 10.1210/mend.10.3.8833652. Mol Endocrinol. 1996. PMID: 8833652
-
Faslodex (ICI 182, 780), a novel estrogen receptor downregulator--future possibilities in breast cancer.J Steroid Biochem Mol Biol. 2001 Dec;79(1-5):209-12. doi: 10.1016/s0960-0760(01)00138-8. J Steroid Biochem Mol Biol. 2001. PMID: 11850227 Review.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
Cited by
-
A transcriptomics model of estrogen action in the ovine fetal hypothalamus: evidence for estrogenic effects of ICI 182,780.Physiol Rep. 2018 Sep;6(18):e13871. doi: 10.14814/phy2.13871. Physiol Rep. 2018. PMID: 30221477 Free PMC article.
-
Membrane-initiated estradiol signaling in immortalized hypothalamic N-38 neurons.Steroids. 2013 Jun;78(6):607-13. doi: 10.1016/j.steroids.2012.12.008. Epub 2013 Jan 5. Steroids. 2013. PMID: 23296142 Free PMC article.
-
Estrogen receptor-alpha 36 mediates mitogenic antiestrogen signaling in ER-negative breast cancer cells.PLoS One. 2012;7(1):e30174. doi: 10.1371/journal.pone.0030174. Epub 2012 Jan 19. PLoS One. 2012. PMID: 22276155 Free PMC article.
-
Reduction of post injury neointima formation due to 17beta-estradiol and phytoestrogen treatment is not influenced by the pure synthetic estrogen receptor antagonist ICI 182,780 in vitro.BMC Cardiovasc Disord. 2002 Aug 6;2:13. doi: 10.1186/1471-2261-2-13. BMC Cardiovasc Disord. 2002. PMID: 12162794 Free PMC article.
-
Okadaic acid induces tau phosphorylation in SH-SY5Y cells in an estrogen-preventable manner.Brain Res. 2010 Jul 23;1345:176-81. doi: 10.1016/j.brainres.2010.04.074. Epub 2010 May 7. Brain Res. 2010. PMID: 20457142 Free PMC article.
References
-
- Limieux P, Fuqua S. J Steroid Biochem Mol Biol. 1996;56:87–91. - PubMed
-
- Luisi B F, Schwabe J W R, Freedman L P. Vitam Horm. 1994;49:1–47. - PubMed
-
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Nordenskjold M, Gustafsson J-A. J Clin Endocrinol Metab. 1997;82:4258–4265. - PubMed
-
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J-A, Nilsson S. Mol Pharmacol. 1998;54:105–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

