A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3)
- PMID: 10725384
- PMCID: PMC16294
- DOI: 10.1073/pnas.97.7.3649
A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3)
Abstract
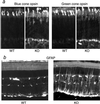
The X-linked RP3 locus codes for retinitis pigmentosa GTPase regulator (RPGR), a protein of unknown function with sequence homology to the guanine nucleotide exchange factor for Ran GTPase. We created an RPGR-deficient murine model by gene knockout. In the mutant mice, cone photoreceptors exhibit ectopic localization of cone opsins in the cell body and synapses and rod photoreceptors have a reduced level of rhodopsin. Subsequently, both cone and rod photoreceptors degenerate. RPGR was found normally localized to the connecting cilia of rod and cone photoreceptors. These data point to a role for RPGR in maintaining the polarized protein distribution across the connecting cilium by facilitating directional transport or restricting redistribution. The function of RPGR is essential for the long-term maintenance of photoreceptor viability.
Figures






References
-
- Berson E L, Rosner B, Simonoff E. Am J Ophthalmol. 1980;89:763–775. - PubMed
-
- Beron E L, Gouras P, Gunkel R D, Myrianthopoulos N C. Arch Ophthalmol. 1969;81:215–225. - PubMed
-
- Jacobson S G, Buraczynska M, Milam A H, Chen C, Jarvalainen M, Fujita R, Wu W, Huang Y, Cideciyan A V, Swaroop A. Invest Ophthalmol Visual Sci. 1997;38:1983–1997. - PubMed
-
- Weleber R G, Butler N S, Murphey W H, Sheffield V C, Stone E M. Arch Ophthalmol. 1997;115:1429–1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

