Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET)
- PMID: 10725388
- PMCID: PMC16300
- DOI: 10.1073/pnas.97.7.3684
Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET)
Abstract
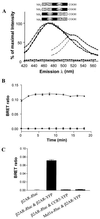
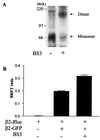
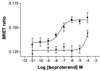
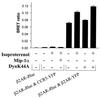
Heptahelical receptors that interact with heterotrimeric G proteins represent the largest family of proteins involved in signal transduction across biological membranes. Although these receptors generally were believed to be monomeric entities, a growing body of evidence suggests that they may form functionally relevant dimers. However, a definitive demonstration of the existence of G protein-coupled receptor (GPCR) dimers at the surface of living cells is still lacking. Here, using bioluminescence resonance energy transfer (BRET), as a protein-protein interaction assay in whole cells, we unambiguously demonstrate that the human beta(2)-adrenergic receptor (beta(2)AR) forms constitutive homodimers when expressed in HEK-293 cells. Receptor stimulation with the hydrophilic agonist isoproterenol led to an increase in the transfer of energy between beta(2)AR molecules genetically fused to the BRET donor (Renilla luciferase) and acceptor (green fluorescent protein), respectively, indicating that the agonist interacts with receptor dimers at the cell surface. Inhibition of receptor internalization did not prevent agonist-promoted BRET, demonstrating that it did not result from clustering of receptors within endosomes. The notion that receptor dimers exist at the cell surface was confirmed further by the observation that BS3, a cell-impermeable cross-linking agent, increased BRET between beta(2)AR molecules. The selectivity of the constitutive interaction was documented by demonstrating that no BRET occurred between the beta(2)AR and two other unrelated GPCR. In contrast, the well characterized agonist-dependent interaction between the beta(2)AR and the regulatory protein beta-arrestin could be monitored by BRET. Taken together, the data demonstrate that GPCR exist as functional dimers in vivo and that BRET-based assays can be used to study both constitutive and hormone-promoted selective protein-protein interactions.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

