The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta
- PMID: 10725390
- PMCID: PMC16313
- DOI: 10.1073/pnas.97.7.3759
The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta
Abstract
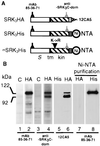
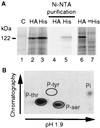
To gain further insight into the mode of action of S-locus receptor kinase (SRK), a receptor-like kinase involved in the self-incompatibility response in Brassica, different recombinant SRK proteins have been expressed in a membranous environment using the insect cell/baculovirus system. Recombinant SRK proteins exhibited properties close to those of the endogenous stigmatic SRK protein and were found to autophosphorylate on serine and threonine residues in insect cell microsomes. Autophosphorylation was constitutive because it did not require the presence of pollen or stigma extracts in the phosphorylation buffer. Phosphorylation was shown to occur in trans, suggesting the existence of constitutive homooligomers of membrane-anchored recombinant SRK. To investigate the physiological relevance of these results, we have examined the oligomeric status of SRK in planta in cross-linking experiments and by velocity sedimentation on sucrose gradients. Our data strongly suggest that SRK is associated both with other SRK molecules and other stigma proteins in nonpollinated flowers. These findings may have important implications for our understanding of self-pollen signaling.
Figures

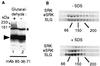
References
-
- De Nettancourt D. Incompatibility in Angiosperms. New York: Springer; 1977.
-
- Nasrallah J B, Kao T-H, Goldberg M L, Nasrallah M E. Nature (London) 1985;318:617–618.
-
- Kandasamy M K, Paolillo D J, Faraday C D, Nasrallah J B, Nasrallah M E. Dev Biol. 1989;134:462–472. - PubMed
-
- Delorme V, Giranton J L, Hatzfeld Y, Friry A, Heizmann P, Ariza M J, Dumas C, Gaude T, Cock J M. Plant J. 1995;7:429–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

