Differential regulation of circadian pacemaker output by separate clock genes in Drosophila
- PMID: 10725392
- PMCID: PMC16287
- DOI: 10.1073/pnas.97.7.3608
Differential regulation of circadian pacemaker output by separate clock genes in Drosophila
Abstract
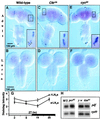
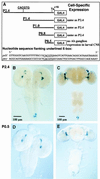
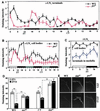
Regulation of the Drosophila pigment-dispersing factor (pdf) gene products was analyzed in wild-type and clock mutants. Mutations in the transcription factors CLOCK and CYCLE severely diminish pdf RNA and neuropeptide (PDF) levels in a single cluster of clock-gene-expressing brain cells, called small ventrolateral neurons (s-LN(v)s). This clock-gene regulation of specific cells does not operate through an E-box found within pdf regulatory sequences. PDF immunoreactivity exhibits daily cycling, but only within terminals of axons projecting from the s-LN(v)s. This posttranslational rhythm is eliminated by period or timeless null mutations, which do not affect PDF staining in cell bodies or pdf mRNA levels. Therefore, within these chronobiologically important neurons, separate elements of the central pacemaking machinery regulate pdf or its product in novel and different ways. Coupled with contemporary results showing a pdf-null mutant to be severely defective in its behavioral rhythmicity, the present results reveal PDF as an important circadian mediator whose expression and function are downstream of the clockworks.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

