Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression
- PMID: 10725400
- PMCID: PMC18105
- DOI: 10.1073/pnas.070047997
Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression
Abstract
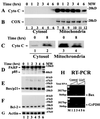
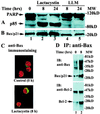
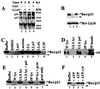
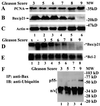
Previously we reported that proteasome inhibitors were able to overcome Bcl-2-mediated protection from apoptosis. Here we show that inhibition of the proteasome activity in Bcl-2-overexpressing cells accumulates the proapoptotic Bax protein to mitochondria/cytoplasm, where it interacts to Bcl-2 protein. This event was followed by release of mitochondrial cytochrome c into the cytosol and activation of caspase-mediated apoptosis. In contrast, proteasome inhibition did not induce any apparent changes in Bcl-2 protein levels. In addition, treatment with a proteasome inhibitor increased levels of ubiquitinated forms of Bax protein, without any effects on Bax mRNA expression. We also established a cell-free Bax degradation assay in which an in vitro-translated, (35)S-labeled Bax protein can be degraded by a tumor cell protein extract, inhibitable by addition of a proteasome inhibitor or depletion of the proteasome or ATP. The Bax degradation activity can be reconstituted in the proteasome-depleted supernatant by addition of a purified 20S proteasome or proteasome-enriched fraction. Finally, by using tissue samples of human prostate adenocarcinoma, we demonstrated that increased levels of Bax degradation correlated well with decreased levels of Bax protein and increased Gleason scores of prostate cancer. Our studies strongly suggest that ubiquitin/proteasome-mediated Bax degradation is a novel survival mechanism in human cancer cells and that selective targeting of this pathway should provide a unique approach for treatment of human cancers, especially those overexpressing Bcl-2.
Figures
Similar articles
-
Inhibition of ubiquitin-proteasome pathway activates a caspase-3-like protease and induces Bcl-2 cleavage in human M-07e leukaemic cells.Biochem J. 1999 May 15;340 ( Pt 1)(Pt 1):127-33. Biochem J. 1999. PMID: 10229667 Free PMC article.
-
mdm2 and bax, downstream mediators of the p53 response, are degraded by the ubiquitin-proteasome pathway.Cell Growth Differ. 1998 Jan;9(1):79-84. Cell Growth Differ. 1998. PMID: 9438391
-
Proteasome inhibitors induce cytochrome c-caspase-3-like protease-mediated apoptosis in cultured cortical neurons.J Neurosci. 2000 Jan 1;20(1):259-65. doi: 10.1523/JNEUROSCI.20-01-00259.2000. J Neurosci. 2000. PMID: 10627603 Free PMC article.
-
The ubiquitin-proteasome pathway and proteasome inhibitors.Med Res Rev. 2001 Jul;21(4):245-73. doi: 10.1002/med.1009. Med Res Rev. 2001. PMID: 11410931 Free PMC article. Review.
-
Proteasomes in apoptosis: villains or guardians?Cell Mol Life Sci. 1999 Dec;56(11-12):908-17. doi: 10.1007/s000180050483. Cell Mol Life Sci. 1999. PMID: 11212325 Free PMC article. Review.
Cited by
-
Expression and bioinformatics analyses show HSP70 complements BCL2 action in oral carcinogenesis.J Oral Biol Craniofac Res. 2022 Sep-Oct;12(5):599-603. doi: 10.1016/j.jobcr.2022.07.009. Epub 2022 Jul 20. J Oral Biol Craniofac Res. 2022. PMID: 36035812 Free PMC article.
-
PKD1 protein is involved in reactive oxygen species-mediated mitochondrial depolarization in cooperation with protein kinase Cδ (PKCδ).J Biol Chem. 2015 Apr 17;290(16):10472-85. doi: 10.1074/jbc.M114.619148. Epub 2015 Mar 10. J Biol Chem. 2015. PMID: 25759386 Free PMC article.
-
The novel combination of sirolimus and bortezomib prevents graft-versus-host disease but maintains the graft-versus-leukemia effect after allogeneic transplantation.Haematologica. 2012 Sep;97(9):1329-37. doi: 10.3324/haematol.2011.058677. Epub 2012 Apr 24. Haematologica. 2012. PMID: 22532520 Free PMC article.
-
The E3 ubiquitin ligase TRIM17 promotes gastric cancer survival and progression via controlling BAX stability and antagonizing apoptosis.Cell Death Differ. 2023 Oct;30(10):2322-2335. doi: 10.1038/s41418-023-01221-1. Epub 2023 Sep 11. Cell Death Differ. 2023. PMID: 37697039 Free PMC article.
-
Measles virus phosphoprotein inhibits apoptosis and enhances clonogenic and migratory properties in HeLa cells.J Biosci. 2019 Mar;44(1):10. J Biosci. 2019. PMID: 30837361
References
-
- Steller H. Science. 1995;267:1445–1462. - PubMed
-
- Green D R, Reed J C. Science. 1998;281:1309–1312. - PubMed
-
- Gross A, McDonnel J M, Korsmeyer S J. Genes Dev. 1999;13:1899–1911. - PubMed
-
- Martin S J, Green D R. Cell. 1995;82:349–352. - PubMed
-
- Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

