The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response
- PMID: 10725405
- PMCID: PMC16247
- DOI: 10.1073/pnas.97.7.3376
The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response
Abstract
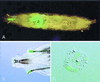
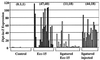
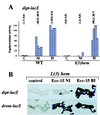
Although Drosophila possesses potent immune responses, little is known about the microbial pathogens that infect Drosophila. We have identified members of the bacterial genus Erwinia that induce the systemic expression of genes encoding antimicrobial peptides in Drosophila larvae after ingestion. These Erwinia strains are phytopathogens and use flies as vectors; our data suggest that these strains have also evolved mechanisms for exploiting their insect vectors as hosts. Erwinia infections induce an antimicrobial response in Drosophila larvae with a preferential expression of antibacterial versus antifungal peptide-encoding genes. Antibacterial peptide gene expression after Erwinia infection is reduced in two Drosophila mutants that have reduced numbers of hemocytes, suggesting that blood cells play a role in regulating Drosophila antimicrobial responses and also illustrating that this Drosophila-Erwinia interaction provides a powerful model for dissecting host-pathogen relationships.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

