Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4
- PMID: 10727445
- PMCID: PMC377464
- DOI: 10.1172/JCI8624
Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4
Abstract
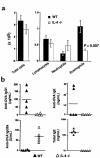
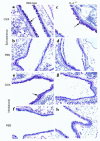
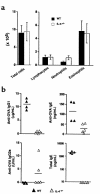
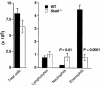
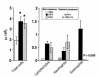
Atopic individuals are predisposed to mounting vigorous Th2-type immune responses to environmental allergens. To determine the factors responsible, animal models that closely mimic natural modes of allergen exposure should prove most informative. Therefore, we investigated the role of IL-4, a known Th2-promoting cytokine, in generation of Th2 responses after exposure of either the skin or airway to soluble protein. Compared with wild-type (WT) mice, IL-4-deficient (IL-4(-/-)) mice showed markedly impaired Th2 activation after primary exposure to inhaled ovalbumin (OVA), with decreased OVA-specific IgG1 and IgE, and significantly fewer eosinophils in bronchoalveolar lavage (BAL) fluid after airway challenge. In contrast, IL-4(-/-) mice initially exposed to epicutaneous (e.c.) OVA mounted Th2 responses equivalent to responses in WT mice, with high numbers of eosinophils in BAL fluid. Because Th2 responses were not induced by e.c. OVA exposure in Stat6(-/-) mice (mice lacking signal transducer and activator of transcription 6), the role of IL-13 was tested. In vivo depletion of IL-13 prevented Th2 responses induced by e.c. OVA exposure in IL-4(-/-) mice. These data demonstrate a marked difference in the IL-4 dependence of Th2 responses generated at two anatomic sites of natural allergen encounter and identify the skin as a particularly potent site for Th2 sensitization.
Figures


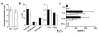
References
-
- van Reijsen FC, et al. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol. 1992;90:184–192. - PubMed
-
- Hamid Q, et al. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–231. - PubMed
-
- Robinson D, et al. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993;92:313–324. - PubMed
-
- Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

