A revised model of platelet aggregation
- PMID: 10727447
- PMCID: PMC377457
- DOI: 10.1172/JCI7569
A revised model of platelet aggregation
Abstract
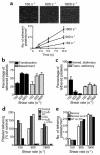
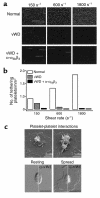
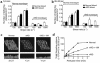
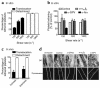
In this study we have examined the mechanism of platelet aggregation under physiological flow conditions using an in vitro flow-based platelet aggregation assay and an in vivo rat thrombosis model. Our studies demonstrate an unexpected complexity to the platelet aggregation process in which platelets in flowing blood continuously tether, translocate, and/or detach from the luminal surface of a growing platelet thrombus at both arterial and venous shear rates. Studies of platelets congenitally deficient in von Willebrand factor (vWf) or integrin alpha(IIb)beta(3) demonstrated a key role for platelet vWf in mediating platelet tethering and translocation, whereas integrin alpha(IIb)beta(3) mediated cell arrest. Platelet aggregation under flow appears to be a multistep process involving: (a) exposure of vWf on the surface of immobilized platelets; (b) a reversible phase of platelet aggregation mediated by the binding of GPIbalpha on the surface of free-flowing platelets to vWf on the surface of immobilized platelets; and (c) an irreversible phase of aggregation dependent on integrin alpha(IIb)beta(3). Studies of platelet thrombus formation in vivo demonstrate that this multistep adhesion mechanism is indispensable for platelet aggregation in arterioles and also appears to promote platelet aggregate formation in venules. Together, our studies demonstrate an important role for platelet vWf in initiating the platelet aggregation process under flow and challenge the currently accepted view that the vWf-GPIbalpha interaction is exclusively involved in initiating platelet aggregation at elevated shear rates.
Figures

Comment in
-
Old concepts and new developments in the study of platelet aggregation.J Clin Invest. 2000 Mar;105(6):699-701. doi: 10.1172/JCI9604. J Clin Invest. 2000. PMID: 10727435 Free PMC article. No abstract available.
References
-
- Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. - PubMed
-
- Tschopp TB, Weiss HJ, Baumgartner HR. Decreased adhesion of platelet to subendothelium in von Willebrand’s disease. J Lab Clin Med. 1973;83:296–300. - PubMed
-
- Weiss HJ, Turitto VT, Baumgartner HR. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. I. Shear rate-dependent decrease of adhesion in von Willebrand’s disease and the Bernard-Soulier syndrome. J Lab Clin Med. 1978;92:750–764. - PubMed
-
- Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen and translocation on von Willebrand factor. Cell. 1996;84:289–297. - PubMed
-
- Holmsen, H. 1986. Platelet responses and metabolism. CRC Press. Boca Raton, FL. pp. 11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

