Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture
- PMID: 10727451
- PMCID: PMC377461
- DOI: 10.1172/JCI8195
Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture
Abstract
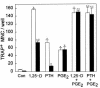
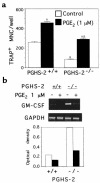
We examined the effect on osteoclast formation of disrupting the prostaglandin G/H synthase genes PGHS-1 and-2. Prostaglandin E(2) (PGE(2)) production was significantly reduced in marrow cultures from mice lacking PGHS-2 (PGHS-2(-/-)) compared with wild-type (PGHS-2(+/+)) cultures. Osteoclast formation, whether stimulated by 1,25-dihydroxyvitamin D(3) (1,25-D) or by parathyroid hormone (PTH), was reduced by 60-70% in PGHS-2(-/-) cultures relative to wild-type cultures, an effect that could be reversed by providing exogenous PGE(2). Cultures from heterozygous mice showed an intermediate response. PGHS inhibitors caused a similar drop in osteoclast formation in wild-type cultures. Co-culture experiments showed that supporting osteoblasts, rather than osteoclast precursors, accounted for the blunted response to 1,25-D and PTH. This lack of response appeared to result from reduced expression of RANK ligand (RANKL) in osteoblasts. We cultured spleen cells with exogenous RANKL and found that osteoclast formation was 50% lower in PGHS-2(-/-) than in wild-type cultures, apparently because the former cells expressed high levels of GM-CSF. Injection of PTH above the calvaria caused hypercalcemia in wild-type but not PGHS-2(-/-) mice. Histological examination of bone from 5-week-old PGHS-2(-/-) mice revealed no abnormalities. Mice lacking PGHS-1 were similar to wild-type mice in all of these parameters. These data suggest that PGHS-2 is not necessary for wild-type bone development but plays a critical role in bone resorption stimulated by 1,25-D and PTH.
Figures


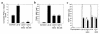




References
-
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:29569–29575. - PubMed
-
- Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266:12866–12872. - PubMed
-
- Reddy ST, Herschman HR. Transcellular prostaglandin production following mast cell activation is mediated by proximal secretory phospholipase A2 and distal prostaglandin synthase 1. J Biol Chem. 1996;271:186–191. - PubMed
-
- Reddy ST, Herschman HR. Prostaglandin synthase-1 and prostaglandin synthase-2 are coupled to distinct phospholipases for the generation of prostaglandin D2 in activated mast cells. J Biol Chem. 1997;272:3231–3237. - PubMed
-
- Chulada PC, Langenbach R. Differential inhibition of murine prostaglandin synthase-1 and -2 by nonsteroidal anti-inflammatory drugs using exogenous and endogenous sources of arachidonic acid. J Pharmacol Exp Ther. 1997;280:606–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

