Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity
- PMID: 10727456
- PMCID: PMC2193122
- DOI: 10.1084/jem.191.6.937
Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity
Abstract
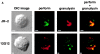
The specificity of immunoglobulins and alpha/beta T cell receptors (TCRs) provides a framework for the molecular basis of antigen recognition. Yet, evolution has preserved a separate lineage of gamma/delta antigen receptors that share characteristics of both immunoglobulins and alpha/beta TCRs but whose antigens remain poorly understood. We now show that T cells of the major tissue gamma/delta T cell subset recognize nonpolymorphic CD1c molecules. These T cells proliferated in response to CD1+ presenter cells, lysed CD1c+ targets, and released T helper type 1 (Th1) cytokines. The CD1c-reactive gamma/delta T cells were cytotoxic and used both perforin- and Fas-mediated cytotoxicity. Moreover, they produced granulysin, an important antimicrobial protein. Recognition of CD1c was TCR mediated, as recognition was transferred by transfection of the gamma/delta TCR. Importantly, all CD1c-reactive gamma/delta T cells express V delta 1 TCRs, the TCR expressed by most tissue gamma/delta T cells. Recognition by this tissue pool of gamma/delta T cells provides the human immune system with the capacity to respond rapidly to nonpolymorphic molecules on professional antigen presenting cells (APCs) in the absence of foreign antigens that may activate or eliminate the APCs. The presence of bactericidal granulysin suggests these cells may directly mediate host defense even before foreign antigen-specific T cells have differentiated.
Figures
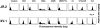





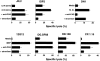



Comment in
-
CD1, tuberculosis, and the evolution of major histocompatibility complex molecules.J Exp Med. 2000 Mar 20;191(6):907-14. doi: 10.1084/jem.191.6.907. J Exp Med. 2000. PMID: 10727453 Free PMC article. Review. No abstract available.
References
-
- Ladel C.H., Blum C., Dreher A., Reifenberg K., Kaufmann S.H.E. Protective role of γ/δ T cells and α/β T cells in tuberculosis. Eur. J. Immunol. 1995;25:2877–2881. - PubMed
-
- Ladel C.H., Hess J., Daugelat S., Mombaerts P., Tonegawa S., Kaufmann S.H.E. Contribution of α/β and γ/δ T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guérinstudies with T cell receptor-deficient mutant mice. Eur. J. Immunol. 1995;25:838–846. - PubMed
-
- D'Souza C.D., Cooper A.M., Frank A.A., Mazzaccaro R.J., Bloom B.R., Orme I.M. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis . J. Immunol. 1997;158:1217–1221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

