Deregulated E2F transcriptional activity in autonomously growing melanoma cells
- PMID: 10727462
- PMCID: PMC2193116
- DOI: 10.1084/jem.191.6.1005
Deregulated E2F transcriptional activity in autonomously growing melanoma cells
Abstract
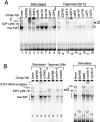
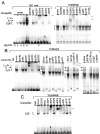
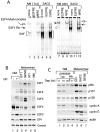
Inactivation of the retinoblastoma tumor suppressor protein (pRb) has been implicated in melanoma cells, but the molecular basis for this phenotype has not yet been elucidated, and the status of additional family members (p107 and p130, together termed pocket proteins) or the consequences on downstream targets such as E2F transcription factors are not known. Because cell cycle progression is dependent on the transcriptional activity of E2F family members (E2F1-E2F6), most of them regulated by suppressive association with pocket proteins, we characterized E2F-pocket protein DNA binding activity in normal versus malignant human melanocytes. By gel shift analysis, we show that in mitogen-dependent normal melanocytes, external growth factors tightly controlled the levels of growth-promoting free E2F DNA binding activity, composed largely of E2F2 and E2F4, and the growth-suppressive E2F4-p130 complexes. In contrast, in melanoma cells, free E2F DNA binding activity (E2F2 and E2F4, to a lesser extent E2F1, E2F3, and occasionally E2F5), was constitutively maintained at high levels independently of external melanocyte mitogens. E2F1 was the only family member more abundant in the melanoma cells compared with normal melanocytes, and the approximately fivefold increase in DNA binding activity could be accounted for mostly by a similar increase in the levels of the dimerization partner DP1. The continuous high expression of cyclin D1, A2, and E, the persistent cyclin-dependent kinase 4 (CDK4) and CDK2 activities, and the presence of hyperphosphorylated forms of pRb, p107, and p130, suggest that melanoma cells acquired the capacity for autonomous growth through inactivation of all three pocket proteins and release of E2F activity, otherwise tightly regulated in normal melanocytes by external growth factors.
Figures



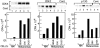

Similar articles
-
Melanoma cell autonomous growth: the Rb/E2F pathway.Cancer Metastasis Rev. 1999;18(3):333-43. doi: 10.1023/a:1006396104073. Cancer Metastasis Rev. 1999. PMID: 10721488 Review.
-
The E2F-family proteins induce distinct cell cycle regulatory factors in p16-arrested, U343 astrocytoma cells.Oncogene. 1998 Aug 20;17(7):867-76. doi: 10.1038/sj.onc.1202008. Oncogene. 1998. PMID: 9780003
-
Activation of a cAMP pathway and induction of melanogenesis correlate with association of p16(INK4) and p27(KIP1) to CDKs, loss of E2F-binding activity, and premature senescence of human melanocytes.Exp Cell Res. 1999 Dec 15;253(2):561-72. doi: 10.1006/excr.1999.4688. Exp Cell Res. 1999. PMID: 10585280
-
Melanin accumulation accelerates melanocyte senescence by a mechanism involving p16INK4a/CDK4/pRB and E2F1.Ann N Y Acad Sci. 2000 Jun;908:71-84. doi: 10.1111/j.1749-6632.2000.tb06637.x. Ann N Y Acad Sci. 2000. PMID: 10911949
-
Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation.Crit Rev Biochem Mol Biol. 1996 Jun;31(3):237-71. doi: 10.3109/10409239609106585. Crit Rev Biochem Mol Biol. 1996. PMID: 8817077 Review.
Cited by
-
HEDGEHOG/GLI-E2F1 axis modulates iASPP expression and function and regulates melanoma cell growth.Cell Death Differ. 2015 Dec;22(12):2006-19. doi: 10.1038/cdd.2015.56. Epub 2015 May 29. Cell Death Differ. 2015. PMID: 26024388 Free PMC article.
-
Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation.PLoS One. 2014 May 7;9(5):e94298. doi: 10.1371/journal.pone.0094298. eCollection 2014. PLoS One. 2014. PMID: 24804719 Free PMC article.
-
Rb-Raf-1 interaction disruptor RRD-251 induces apoptosis in metastatic melanoma cells and synergizes with dacarbazine.Mol Cancer Ther. 2010 Dec;9(12):3330-41. doi: 10.1158/1535-7163.MCT-10-0442. Epub 2010 Dec 7. Mol Cancer Ther. 2010. PMID: 21139044 Free PMC article.
-
Haplotype-based analysis resolves missing heritability in oculocutaneous albinism type 1B.Am J Hum Genet. 2023 Jul 6;110(7):1123-1137. doi: 10.1016/j.ajhg.2023.05.012. Epub 2023 Jun 15. Am J Hum Genet. 2023. PMID: 37327787 Free PMC article.
-
Genomic analysis reveals distinct mechanisms and functional classes of SOX10-regulated genes in melanocytes.Hum Mol Genet. 2015 Oct 1;24(19):5433-50. doi: 10.1093/hmg/ddv267. Epub 2015 Jul 23. Hum Mol Genet. 2015. PMID: 26206884 Free PMC article.
References
-
- Halaban R. Melanoma cell autonomous growththe Rb/E2F pathway. Cancer Metastasis Rev. 1999;8:333–343. - PubMed
-
- Halaban R. Growth factors and melanomas. Semin. Oncol. 1996;23:673–681. - PubMed
-
- Rodeck U., Melber K., Kath R., Menssen H.D., Varello M., Atkinson B., Herlyn M. Constitutive expression of multiple growth factor genes by melanoma cells but not normal melanocytes. J. Investig. Dermatol. 1991;97:20–26. - PubMed
-
- Rodeck U. Growth factor independence and growth regulatory pathways in human melanoma development. Cancer Metastasis Rev. 1993;12:219–226. - PubMed
-
- Bartkova J., Lukas J., Guldberg P., Alsner J., Kirkin A.F., Zeuthen J., Bartek J. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 1996;56:5475–5483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

